How prevalent is Bartonella in people who have Lyme disease?
At a meeting of the federal Tick-Borne Disease Working Group on March 1, Ben Beard, PhD of the CDC made a highly significant statement that passed without remark at the time.
Beard’s statement was in reply to a comment by Monica Embers, PhD, also a member of the working group. Embers noted that several slides from Beard’s Clinical Presentation and Pathogenesis subcommittee mentioned neuropsychiatric illness and neuropathic manifestations of Lyme disease.
“We’re seeing a lot more neuropsychiatric disease associated with Bartonella,” said Embers. “I’m wanting to hear more about your thought process and your recommendation with respect to bartonellosis?”
Bartonella’s “significant impact”
Beard replied: “In my view Bartonella is ubiquitous. There are multiple different Bartonella species. A lot of people are exposed to cats and fleas, and Bartonella henselae–or cat scratch disease–is pretty common. Our group looked at it as an illness that is associated with people with other tick-borne illnesses. Not necessarily agreeing that it’s tick-borne—for me the jury is still out for that—but I’m perfectly convinced that it is very common, and that it may be confounding the diagnosis, and that it is an important co-infection. We need not get side-tracked on whether or not it’s tick-borne. We need to agree that it’s a common infection, commonly seen in patients with other illnesses, and it can have a significant impact on clinical outcome and presentation.”
This is actually a showstopper of a comment.
The CDC has long declined to categorize bartonellosis as tick-borne and has not considered it a co-infection of Lyme.
Even today, the CDC website states: “Ticks may carry some species of Bartonella bacteria, but there is currently no causal evidence that ticks can transmit Bartonella infection to people through their bites.”
Yet, as Beard observed, Bartonella is very common in people with Lyme disease.
What the data says
In MyLymeData, LymeDisease.org’s patient-led research project, 60% of patients with chronic symptoms of Lyme disease report co-infections. A previously published LymeDisease.org survey of over 3,000 patients found that over 50% had co-infections, with 30% of patients reporting two or more. Bartonella (28%) was the second most commonly reported co-infection associated with chronic Lyme disease. (Johnson, L., et al., 2014)
Bartonella does not respond to standard treatment for Lyme disease, and it is notoriously difficult to detect through standard tests. Moreover, Bartonella is not included in standard surveillance testing for ticks, and cases of the disease are not tracked by the CDC
Which leads me to the elephant in the room: nobody knows how many cases of bartonellosis there are in the US—or anywhere else for that matter.
What is bartonellosis?
Bartonellosis is caused by one of many species of the bacterium Bartonella. It is harbored in wild and domestic animals, and can be transmitted to humans through a number of different pathways including fleas, flies, lice, animal bites, animal scratches, ticks, bedbugs, and possibly through maternal fetal transmission. (Maggi RG, et al., 2015; Reis C, et al., 2011)
First identified in 1990, Bartonella henselae bacteria is the most common cause of bartonellosis in humans. Bartonella henselae infection, also called cat scratch disease, is frequently caused by flea bites or the scratch of an infected cat. The primary reservoirs for B. henselae across the world are domestic and stray cats, and the primary vector is the cat flea (ctenophalides felis). (Breitschwerdt, E.B., 2017)
Prior to 1990, there were only two diseases known to be caused by Bartonella bacteria. One was “Carrion’s disease,” endemic to parts of South America, caused by Bartonella bacilliformis. The other was “trench fever,” which infected many soldiers during World War I, caused by Bartonella quintana. Though the illness was first described in 1915, Bartonella quintana was not molecularly identified as its cause until 1961. (Breitschwerdt, E.B., 2017)
We now know that these bacteria have been infecting humans for thousands of years. Researchers discovered Bartonella quintana in a 4,000-year-old human tooth in France. (Drancourt M., et al., 2005)
Today, at least 40 different species of Bartonella have been identified.About half of them are known to cause symptoms in humans or animals.
Bartonella is a stealth pathogen
At a recent conference, Dr. Ed Breitschwerdt, DVM, a leading expert in the field, explained how Bartonella can invade and “literally affect every system in the body.” This includes the: cutaneous, muscular, skeletal, endocrine, cardiovascular and nervous systems.
He reviewed several recent studies implicating Bartonella infection in the brain in relation to several neuropsychiatric and autoimmune manifestations.
According to Breitschwerdt, these bacteria are extremely difficult to find in humans because they are slow growing and can hide within cells.
He explained how Bartonella, which are intracellular bacteria, have the ability to:
- invade red blood cells, wall themselves off, and hide from the immune system (immune evasion)
- migrate into the nervous system via macrophages (Trojan horse)
- penetrate the blood brain barrier via endothelial cells and pericytes
- persist within the brain via microglial cells.
Considering the number of different species and different methods of contracting Bartonella, Dr. Breitschwerdt ponders, “Is Bartonellosis a modern-day hidden epidemic?” (Breitschwerdt E.B., 2014)
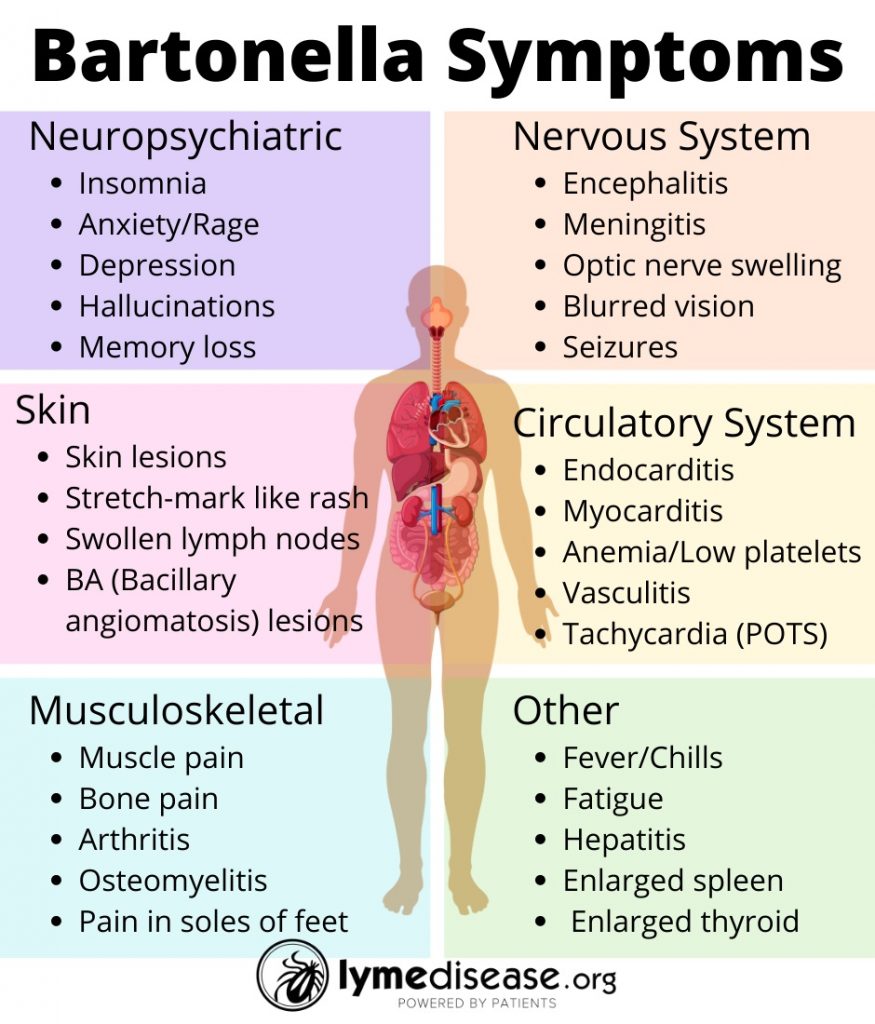
Symptoms of bartonellosis
The symptoms of bartonellosis can range from mild to life-threatening, depending on the Bartonella species and the health of those infected. Furthermore, a growing body of evidence links Bartonella to neuropsychological symptoms.
The most commonly reported neurological symptoms include sleep disorders, mental confusion, memory loss, brain fog, irritability, rage, anxiety, panic attacks, depression, migraines, tremors, hallucinations, psychosis and postural orthostatic tachycardia (POTS).
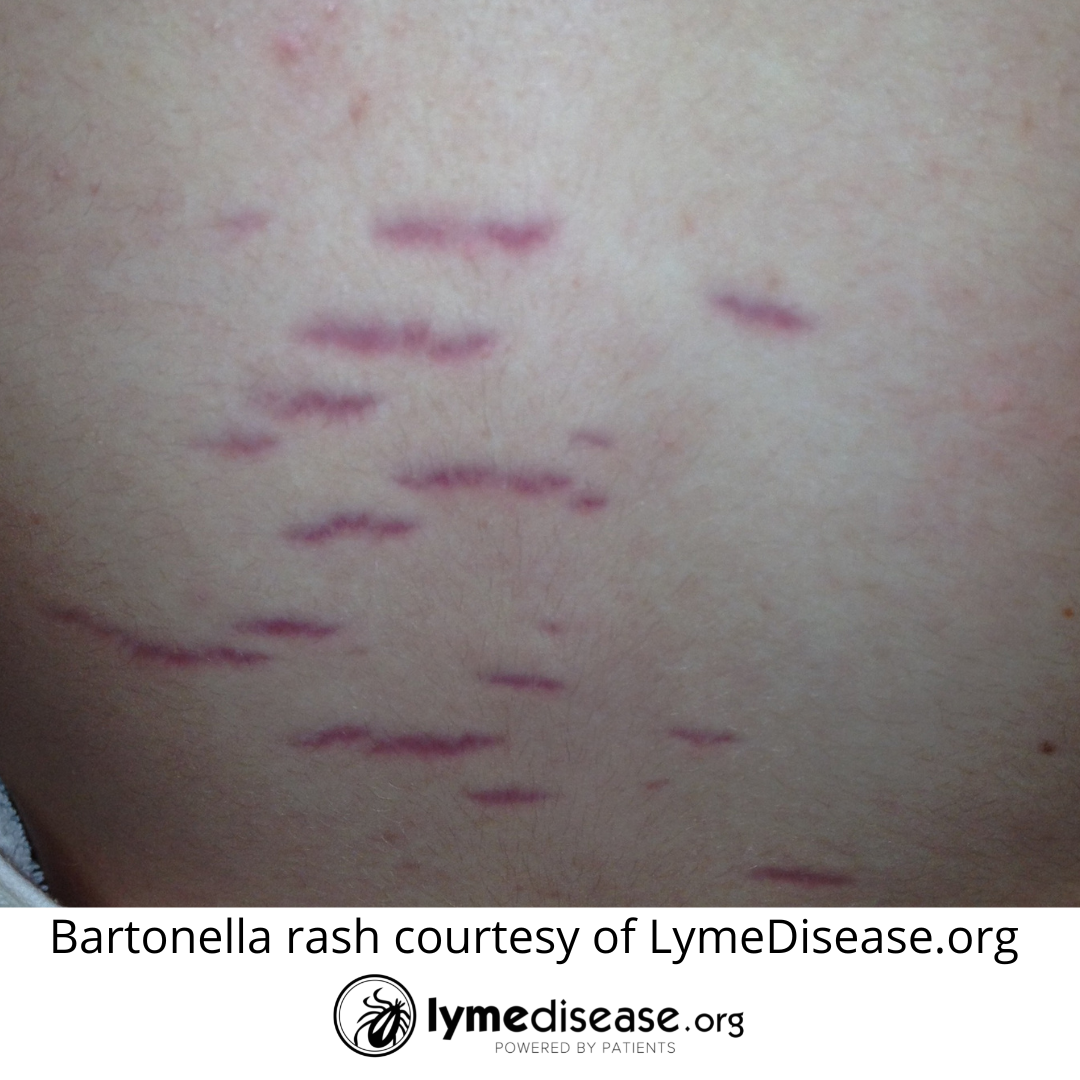
Additional symptoms common to bartonellosis are swollen lymph nodes (especially around the head, neck and arm pits), bone pain (especially shins), pain in the soles of the feet, low grade fever in the morning, night sweats, tender nodules along the extremities, gastrointestinal pain, and skin markings (striae) that resemble stretch marks.
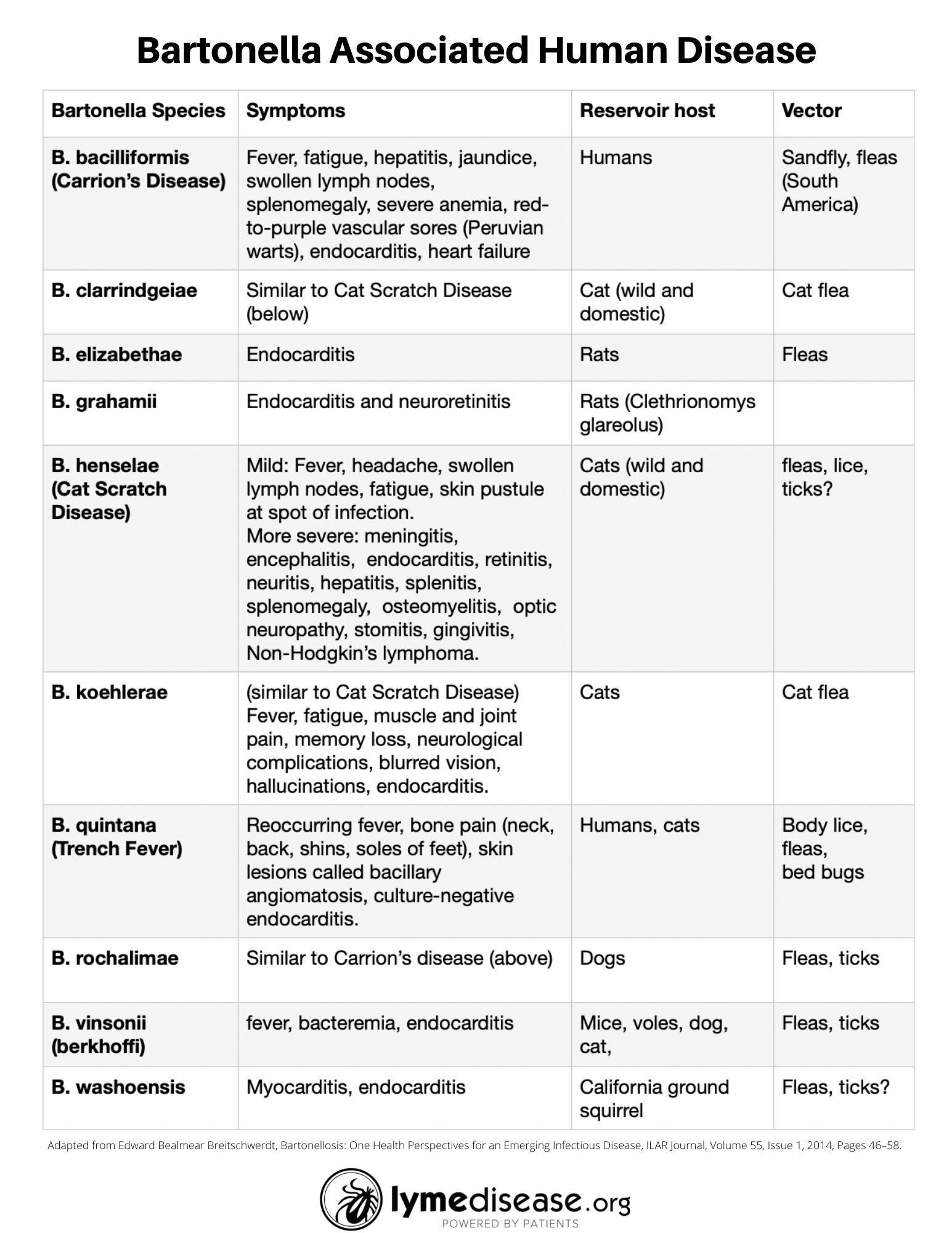
The table below lists the known species of Bartonella associated with human disease, the most common symptoms as well as the reservoir host and vector.
How a stealth pathogen may prolong your chronic illness
In individuals with strong immune systems, Bartonella infection is often mild or asymptomatic. However, in those with an impaired immune system, Bartonella can wreak havoc on the body.
In fact, Bartonella henselae was discovered in the 1990s during the AIDS epidemic. Because the HIV virus causes an acquired immune deficiency, these patients were extremely susceptible to new infections and reactivation of latent infections. In this patient population, Bartonella caused a distinctive skin lesion called bacillary angiomatosis (BA), and a type of liver disease called peliosis hepatis. (Breitschwerdt, E.B., 2017)
Advanced, disseminated disease is more likely to occur in immunocompromised patients or those taking immunosuppressive drugs. Without proper treatment, the infection can spread systemically throughout the body. The result is sometimes fatal.
When the co-infection becomes the main infection
Data from multiple animal studies shows that Borrelia burgdorferi suppresses the immune system. (Buffen K, et al., 2016; Tracy KE, Baumgarth N., 2017)
This makes me wonder. How many people with chronic Lyme disease had a latent Bartonella infection that was re-activated when their immune system became impaired?
I believe this was the case with my daughter. We live on a farm with lots of animals, including cats. Veterinarians, cat owners, and people who live or work on farms are at increased risk for Bartonella.
It wasn’t until my child became deathly ill after contracting Ehrlichia chaffeensis that her Bartonella symptoms began.
The symptoms that stood out were the constant migraine/headache, memory loss, bone pain, painful soles of feet, relapsing fever, insomnia, nighttime hallucinations that made everything look like Whoville, POTS, skin marks (striae) that resembled stretch marks, swollen lymph nodes, and an immune system so impaired it led to a temporary misdiagnosis of HIV. What a horrific experience for all of us!
Diagnosis & Treatment
Because Bartonella may hide inside of cells and only emerge periodically, you may need to test multiple times to find a confirmatory diagnosis. And in patients who are immunocompromised, the test may not turn positive until after treatment has begun.
Research led by Ricardo Maggi, Ed Breitschwerdt and colleagues has led to the development of a new digital PCR that is much more sensitive to Bartonella. Even still, Dr. Maggi recommends running multiple types of tests (IFA serology, PCR, culture, and microscopy).
According to Dr. Joseph Burrascano, one should consider bartonellosis when symptoms persist after treatment for Lyme disease. Especially when the neurological symptoms are out of proportion to the common symptoms of disseminated Lyme disease.
Just as with Lyme disease, the longer Bartonella goes untreated, the more difficult it is to treat. Furthermore, the standard treatment for Lyme (doxycycline) is ineffective against Bart. As Dr. Breitschwerdt famously said, “You cannot float humans or horses in enough doxycycline to kill this bacteria.”
According to the CDC: “A number of antibiotics are effective against Bartonella infections, including azithromycin, penicillins, tetracyclines, cephalosporins, aminoglycosides, and macrolides. More than one antibiotic is often used. Consult with an expert in infectious diseases regarding treatment options.”
Dr. Burrascano says, treating Bartonella-like organisms “can be difficult, as drug resistance can rapidly develop to macrolides and fluoroquinolones when used as a single agent and solo courses of tetracyclines are ineffective.”
Moving forward with Bartonella research
In 2021, a new Bartonella Research Consortium was formed with a $4.8 million grant from The Steven & Alexandra Cohen Foundation.
The consortium includes Ed Breitschwerdt and Ricardo Maggi of North Carolina State University, Monica Embers of Tulane University, and Timothy Haystead of Duke University, who is continuing the work of the late Dr. Neal Spector.
The team is actively working towards creating a targeted treatment for bartonellosis and quickly getting the drug to the marketplace for use in both animals and humans.
It’s time medicine moves beyond the one-pathogen-one-disease model. Let’s face it, ticks are full of toxic soup. Because each pathogen interacts with the host in unique ways, extensive research is needed to understand all factors surrounding co-infections and Lyme disease. (Moutailler S, et al., 2016)
Understanding the complex nature of these pathogens, how they impact the immune system, and how other bacterial and viral factors shape illness, will be key in improving public health. (Cheslock, M. A., & Embers, M. E., 2019)
It’s time for the CDC, NIH, HHS, the Tick-Borne Disease Working Group and other researchers to start looking deeper into the prevalence of Bartonella infections–not just in patients with Lyme disease but in all patients with poorly-defined chronic illnesses.
Resources
More information about testing/diagnosis of Bartonellosis see:
- Galaxy: From Cat Scratch Disease to Bartonellosis
- IGeneX: Bartonella for clinicians
- Lyme Resource Center: Bartonella in Chronic Infection, Dr. Maggi
Free Bartonella CME Course:
LymeSci is written by Lonnie Marcum, a Licensed Physical Therapist and mother of a daughter with Lyme. She has served two terms on a subcommittee of the federal Tick-Borne Disease Working Group. Follow her on Twitter: @LonnieRhea Email her at: lmarcum@lymedisease.org.
References
Centers for Disease Control and Prevention (CDC) Bartonella Infection
Recap of the TBDWG Meeting Feb 20 – March 1, 2022:
Day 1 https://www.lymedisease.org/tbdwg-feb28-live-tweets/, Day 2 https://www.lymedisease.org/2nd-day-summary-tbdwg/
Breitschwerdt E.B., (2014) Bartonellosis: One Health Perspectives for an Emerging Infectious Disease, ILAR Journal, 55 (1) 2014, 46–58. https://doi.org/10.1093/ilar/ilu015
Breitschwerdt, E.B. (2017) Bartonellosis, one health and all creatures great and small. Vet. Dermatol. 2017, 28, 96-e21. doi: 10.1111/vde.12413.
Buffen K, Oosting M, Li Y, Kanneganti TD, Netea MG, Joosten LA. (2016) Autophagy suppresses host adaptive immune responses toward Borrelia burgdorferi. J Leukoc Biol. 100(3):589-98. doi: 10.1189/jlb.4A0715-331R.
Cheslock, M. A., & Embers, M. E. (2019). Human Bartonellosis: An Underappreciated Public Health Problem?. Tropical medicine and infectious disease, 4(2): 69. https://doi.org/10.3390/tropicalmed4020069
Coutte L, Botkin DJ, Gao L, Norris SJ. (2009) Detailed analysis of sequence changes occurring during vlsE antigenic variation in the mouse model of Borrelia burgdorferi infection. PLoS Pathog. 5(2):e1000293. doi: 10.1371/journal.ppat.1000293.
Drancourt M., Tran-Hung, L., Courtin J., de Lumley H., Raoult D., (2005) Bartonella quintana in a 4000-Year-Old Human Tooth, The Journal of Infectious Diseases, 191(4): 607–611. https://doi.org/10.1086/427041
Johnson, L., Wilcox, S., Mankoff, J. and Stricker, RB. (2014) Severity of Chronic Lyme Disease Compared to Other Chronic Conditions: A Quality of Life Survey. PeerJ, DOI 10.7717/peerj.322. (Open access.)
Maggi RG, Balakrishnan N, Bradley JM, Breitschwerdt EB. (2015) Infection with Bartonella henselae in a Danish family. J Clin Microbiol. 53(5):1556-61. doi: 10.1128/JCM.02974-14.
Moutailler S, Valiente Moro C, Vaumourin E, Michelet L, Tran FH, Devillers E, Cosson JF, Gasqui P, Van VT, Mavingui P, Vourc’h G, Vayssier-Taussat M. (2016) Co-infection of Ticks: The Rule Rather Than the Exception. PLoS Negl Trop Dis. 17;10(3):e0004539. doi: 10.1371/journal.pntd.0004539.
Reis C, Cote M, Le Rhun D, Lecuelle B, Levin ML, Vayssier-Taussat M, Bonnet SI. (2011) Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlesii. PLoS Negl Trop Dis. doi: 10.1371/journal.pntd.0001186.
Sperling J, Middelveen M, Klein D, Sperling F. (2012) Evolving perspectives on lyme borreliosis in Canada. Open Neurol J. 6:94-103. doi: 10.2174/1874205X01206010094.
Tracy KE, Baumgarth N. (2017) Borrelia burgdorferi Manipulates Innate and Adaptive Immunity to Establish Persistence in Rodent Reservoir Hosts. Front Immunol. 20(8):116. doi: 10.3389/fimmu.2017.00116.




















We invite you to comment on our Facebook page.
Visit LymeDisease.org Facebook Page