IGeneX to discontinue Western blots in favor of ImmunoBlot tests
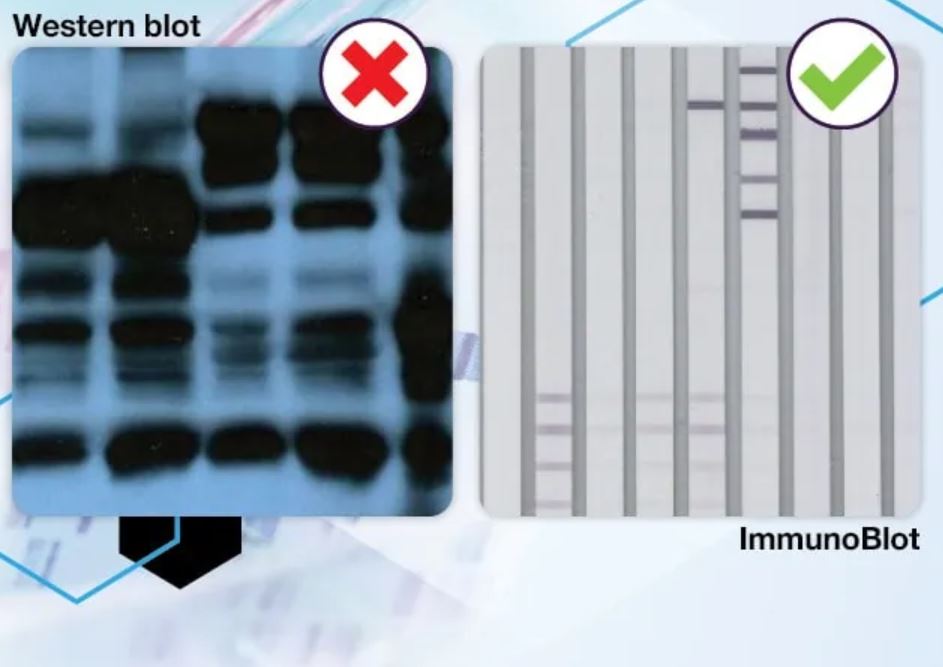
As of Jan. 1, 2024, IGeneX will no longer offer the Western blot test for Lyme disease. It will be replaced by the ImmunBlot.
The company says the ImmunoBlot differs from the Western blot in two key ways.
“First, it looks for multiple pathogens, instead of one with the Western blot. And second, it uses recombinant proteins instead of proteins from natural sources, leading to a more specific test.”
The website goes on to say: “Common Lyme two-tier testing has a sensitivity of just above 50% because it detects only one Borrelia species, B. burgdorferi B31. The IGeneX ImmunoBlot detects antibodies to nine different species of Borrelia: B. burgdorferi B31, B. burgdorferi 297, B. californiensis, B. mayonii, B. afzelii, B. garinii, B. spielmanii, B. bissettii, and B. valaisianaa.”
The company offers Immunoblots for four diseases: Lyme, tick-borne relapsing fever, Bartonella, and Babesia.




















We invite you to comment on our Facebook page.
Visit LymeDisease.org Facebook Page