Why compounding pharmacies matter and why you should care

By Crystal A. Frost, PhD
Compounding pharmacies in California are under a slow-motion attack, and the consequences for patients could be devastating.
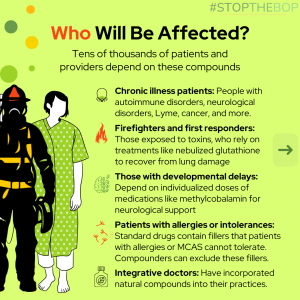
The California State Board of Pharmacy (BOP) is attempting to push through dangerous regulations that threaten to severely restrict access to numerous sterile compounded treatments, including glutathione, NAD+, ALA, and methylcobalamin (vitamin B12), relied upon by thousands of patients.
Stop The BOP, a movement formed this past summer, is dedicated to protecting patient access to these compounded medications. As one of its two leaders, this fight is incredibly personal for me. I suffer from neurological Lyme disease and depend on several of these treatments to maintain my quality of life. I’m grateful to LymeDisease.org for their efforts in alerting the Lyme community about these harmful regulations.
Before diving into the specifics of these regulatory threats, it’s important to understand why compounding pharmacies are so vital and why their survival matters to so many.
What are compounding pharmacies?
Compounding is a specialized form of pharmacy that allows pharmacists to mix active ingredients to create custom doses or formulations not available through mass-produced drugs.
These independent pharmacies are lifelines for those who can’t tolerate or benefit from one-size-fits-all medications. Here are just a few services compounding pharmacies provide:
- Custom dosages for children, seniors, or individuals with unique medical needs.
- Allergen removal for patients sensitive to dyes or fillers in commercial medications.
- Alternative formulations like liquid, topical, nebulized (inhalable), or injectable therapies for patients unable to swallow pills.
- Bio-identical hormones, peptide therapies, and sterile vitamin and mineral preparations to treat nutritional deficiencies.
- Natural compounds that aren’t FDA approved and thus are unavailable anywhere else (i.e. methyl B12 shots, glutathione)
- Filling prescriptions that are otherwise unavailable due to drug shortages.
Compounding pharmacies are divided into two categories: 503A and 503B (also called outsourcing facilities). Both sterile and non-sterile versions exist in each category.
Many treatments are only available through compounding pharmacies, which makes them crucial for vulnerable patient populations when other treatments fail. Mainstream pharmacies can only sell mass-produced drugs, making compounding a necessity for many.
Regulatory threats by the California Board of Pharmacy (BOP)
The BOP’s actions have reached an alarming level of overreach. To give some context, last year, the BOP banned regular pharmacies from flavoring medications—a practice relied upon by children and some elderly patients who cannot swallow pills.
The BOP decided this could only be done by compounding pharmacies while also taking actions that serve to drive compounding pharmacies out of the state. The regulations were so poorly written that it inadvertently banned veterinary pharmacies from making beef-flavored medications for dogs.
Now, the Board’s latest proposed regulations on pharmacy compounding could effectively end patient access to a long list of widely used treatments: Category 1 sterile compounds on the 503A bulks list. This list includes methylcobalamin, glutathione, NAD+, ALA, and dozens more. Every compound on this list has passed FDA safety reviews under an interim policy, making them safe and legal.
 Why does the BOP want to block access?
Why does the BOP want to block access?
Unfortunately, the board has not offered any scientific or safety-based reasons for restricting access to these treatments.
Their main argument has been that these compounds “don’t have a USP drug monograph,” which they equate to being unapproved by the FDA. However, there is no law requiring FDA approval or USP drug monographs for compounded medications, and the FDA approval process costs billions of dollars, making it infeasible for non-patentable natural compounds.
As for safety? USP Chapter 797 already guarantees safety. Stop The BOP is asking the Board to simply adopt these scientifically backed standards used across the U.S. rather than attempting to impose their own nonsensical standards.
The proposed regulations have been unanimously opposed at every public hearing and board meeting, including by the likes of traditional chain pharmacies like Walgreens.
Consequences if these regulations pass
If these regulations are implemented, here’s what we could face:
Redundant Testing Requirements: The cost of compounded medicines will skyrocket, pricing out vulnerable patients.
Regulatory “Land Mines”: The proposed regulatory text is intentionally vague, making it easy for pharmacists to accidentally violate the rules and face citations or license suspensions.
Solidifying Underground Regulation: The BOP has already been enforcing underground regulations, taking disciplinary action against pharmacies for compounding these legal Category 1 substances. These regulations would cement those unwritten rules, putting the final nail in the coffin for many compounding pharmacies and patients who depend on these treatments.
How are they getting away with this?
The California Board of Pharmacy operates with what appears to be unchecked power as the systems in place meant to protect citizens are failing. Here are five reasons why:
Administrative Law Judges (ALJs) can’t enforce their decisions. This means that, even when the BOP loses a case, they can still take disciplinary action against pharmacies. (The BOP has lost all eight lawsuits related to glutathione and methylcobalamin production so far.)
The Department of Consumer Affairs (DCA), which is supposed to oversee the BOP, is turning a blind eye (for now – we hope that changes soon).
Pharmacies are scared to confront the Board due to its retaliatory nature and power to suspend and revoke licenses without oversight.
Assembly Bill 973 signed by Newsom in 2019 allowed the BOP to exceed federal standards in regulating compounding but does not authorize them to enforce non-existent regulations, which they’re doing anyway.
Fines from citations go directly back into the BOP’s budget, incentivizing them to penalize more pharmacies.
Help us protect compounding pharmacies
Stop The BOP is fighting to protect and restore access to these vital compounded medications, but we need your help! Join us in demanding that the BOP adopt USP Chapter 797 and stop restricting access to life-sustaining care.
Without access to compounding pharmacies, thousands of patients would lose life-saving treatments. Your voice matters, and together we can make a difference!
Here’s how you can help:
- Sign and share the petition.
- Send us your story (stopthebop.com/contact or DM us on instagram @stop.thebop)
- Alert your representatives! Templates and instructions available at stopthebop.com/actnow
- Donate at stopthebop.com/donate. This movement is entirely a volunteer effort, and 100% of donations are going directly to our outreach efforts.
- Post using #StopTheBOP and help us spread the word! (Don’t forget to tag us!)
- Give a public comment at the Board of Pharmacy meeting on November 6-7 in San Diego or via Webex. (Updated: the next meeting is March 6.) Access links, prompts, address and more are available at stopthebop.com/actnow. Don’t hesitate to DM us or email me if you have questions at crystal@stopthebop.com. Your participation means the world to us, and we want to make that process as easy as possible for you!
Let’s make our voices heard and protect patient access to critical treatments!
See also:
Why patients fight to protect access to B12 shots as methylcobalamin
Future of IV glutathione and B-12 shots still unsettled in California
Crystal A. Frost, PhD, is Southern California leader of Stop The BOP.






















We invite you to comment on our Facebook page.
Visit LymeDisease.org Facebook Page