Congressional update: Lyme and tick-borne disease appropriations

The Center for Lyme Action (CLA), a nonprofit based in Washington, D.C., lobbies on behalf of the Lyme community. Its goal is to expand federal funding for Lyme disease and other tick-borne illnesses. Here’s CLA’s latest message about what’s happening in Congress.
Dear Lyme Advocate:
Congratulations! We are making great progress, nearly tripling federal Lyme and tick-borne disease funding in the last three years. A special thank you for your individual advocacy. Every person counts!
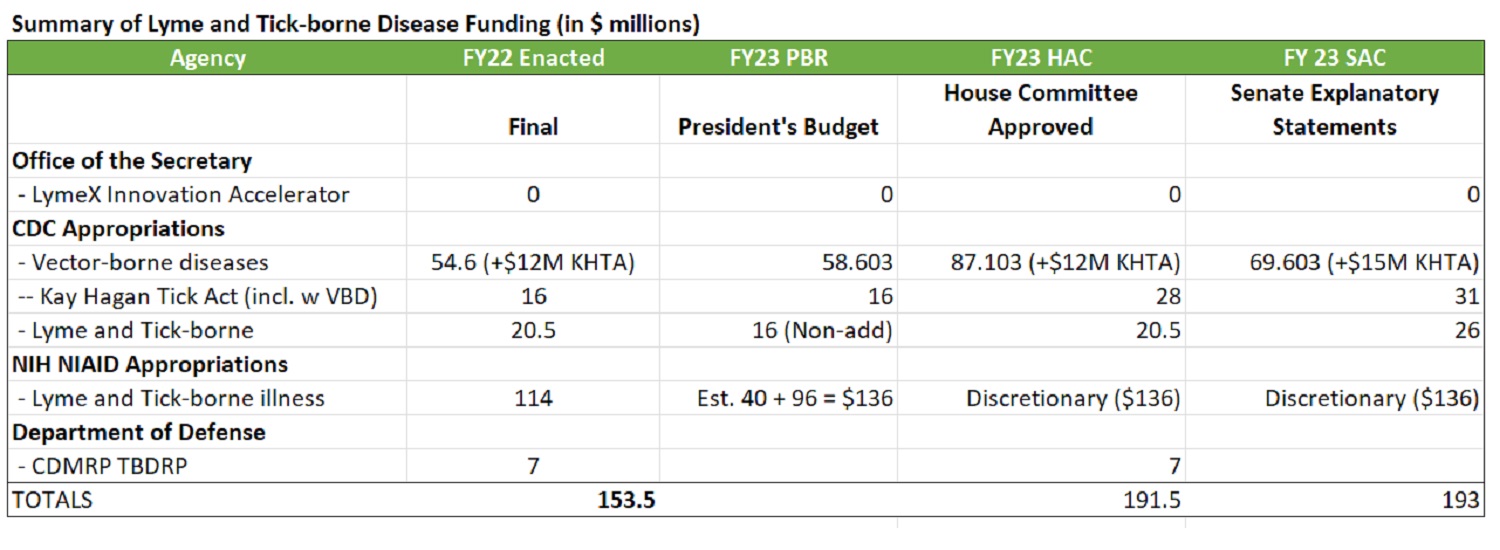
Below is an update on where we stand with the Fiscal Year 2023 appropriations for Lyme and tick-borne disease. Both the House and Senate Appropriations Committees have provided federal funding marks.
The House Appropriations Committee passed the Labor HHS and Defense Appropriations bills out of the full Committee, but neither bill has made it to the House floor.
The Senate Appropriations Committee Democrats provided Explanatory Statements for both Labor-HHS and Defense without a Committee vote. House and Senate negotiators will use those respective versions of the two bills to negotiate a final compromise bill.
In the mean time, last week the House and Senate passed a Continuing Resolution or CR to fund the government through December 16, 2022 at Fiscal Year 2022 levels with some negotiated exceptions.
The House Appropriations Committee approved the following for Lyme and tick-borne disease:
- + $12M for a total of $28M for the Kay Hagan Tick Act
- $20.5M for CDC Lyme and tick-borne diseases
- $7M for Defense CDMRP
The Senate provided Explanatory Statements with the following recommendations:
- + $5.5M increase for the CDC Lyme program for a total amount of $26M
- + $15M increase for the Kay Hagan Tick Act for a total of $31M
What’s next
House and Senate negotiators are meeting to hammer out the final appropriations amounts for Fiscal Year 2023. We don’t expect any final votes until at least after the midterm elections in November. We will keep you updated on any new developments.
Thank you for your continued support and stay tuned! More to come!
Best regards,
Bonnie Crater, Jeff Crater, and Meredith Faucette
P.S. Want more detail? Please see the information below including a summary table and specific details.
CDC
P. 76
Vector-Borne Diseases.—The Committee includes an increase of $12,000,000 for enhanced vector-borne disease activities, including Lyme disease and tick-borne diseases. The Committee urges CDC to increase provider and public awareness of Lyme and known tick-borne diseases (TBD) in differential diagnoses, to practice shared decision making, to be aware of the existence of two sets of differing Lyme Disease Clinical Guidelines, and to encourage the public to take preventive measures. The Committee requests an update in the fiscal year 2024 Congressional Budget Justification on the use of advanced and emerging technologies for the development of improved diagnostics, including a timeline on when improved diagnostics may become commercially available for Lyme disease. In addition, the Committee notes that the pandemic response necessitated the disruption of mosquito control and abatement efforts by many State and local health departments and notes the importance of continuing mosquito prevention efforts. The Committee is aware of the ongoing challenges faced by the U.S. territories in the Caribbean and the Pacific regarding control and management of vector-borne diseases. The Committee urges CDC to support the training and research needs of the U.S. territories and encourages the use of the Mosquito Abatement for Safety and Health Program to provide grants and technical assistance to States and political subdivisions to prevent and control mosquito-borne diseases. In addition, the Committee requests CDC, in consultation with other appropriate agencies, to provide information in the fiscal year 2024 Congressional Budget Justification on the ecological structure and epidemiological factors that must be known and monitored to estimate the mosquito-borne infectious disease outbreak risk.
P. 110
Lyme Disease.—The Committee recognizes that there have been only a small number of clinical trials involving Lyme disease, which lacks a gold standard test, and that those trials have involved a relatively small number of patients. For other diseases, high quality multi-site trials involving robust number of well-characterized patients have been considered essential to facilitate advancements in the development of more effective treatments and improved outcomes. Because of the clear neurological dysfunction of Lyme disease and the existence of the Network for Excellence in Neuroscience Clinical Trials, the Committee encourages NINDS to evaluate how it may contribute to improvements in tools to manage Lyme disease.
NIH
P. 114
Tick-Borne Disease Research.—The Committee is supportive of the NIH Strategic Plan for Tick-borne Disease Research published in 2019. The Committee encourages NIAID to increase efforts to understand causes of the increase in tick-borne diseases, to support research on tick-borne tularemia (Francisella tularensis), and to determine whether information learned on ways that ticks respond to bacterial infections offer avenues to thwart tick infections in humans.
P. 148
Lyme and Other Tick-Borne Diseases.—The Committee encourages NIH to hold a public workshop on the molecular mechanisms that Borrelia burgdorferi (Bb) employs to evade the human immune system, the human immune responses and consequences of Bb infection, and how these mechanisms and responses may influence the effectiveness of antibiotics. The Committee recognizes that there have been only a small number of clinical trials involving Lyme disease, which lacks a gold standard test, and that those trials have involved a relatively small number of patients. Because of the clear neurological dysfunction of Lyme disease and the existence of the Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT), the Committee encourages NINDS to evaluate how it may contribute to improvements in tools to manage Lyme disease. The Committee asks NIH to consider the value of establishing a work group on long-term, not well understood outcomes for different diseases with similar long-term sequelae, particularly SARS–CoV–2 infection and Lyme disease, taking into account the input of patients not fully recovered from these infections and who offer experiences and insights, such as called for in RECOVER. Finally, the Committee encourages NIH to intensify research on adverse outcomes related to Lyme disease during pregnancy and to continue to participate with Lyme advocacy organizations on these issues.
LymeX
p. 221
LymeX Innovation Accelerator.—The Committee commends the Office of the Secretary and its Chief Technology Officer for the Lyme Innovation Initiative, launched November 2018, and the LymeX Innovation Accelerator announced in October 2020. LymeX is a $25,000,000 public-private partnership between HHS and the Steven & Alexandra Cohen Foundation to accelerate innovation in prevention, diagnostics, and treatments for Lyme and other tick-borne diseases.
HOUSE OF REPRESENTATIVES DEPARTMENT OF DEFENSE FY23 APPROPRIATIONS BILL REPORT, 2023
CHRONIC DISEASE PREVENTION AND HEALTH PROMOTION
Appropriation, Fiscal year 2022 ………………………………………………..$1,338,664,000
Budget request, Fiscal year 2023 ………………………………………………… 1,612,264,000
Committee Recommendation …………………………………………………….. 1,601,914,000
Change from enacted level ………………………………………………………… .+263,250,000
Change from budget request ……………………………………………… ………….-10,350,000
p. 76
EMERGING AND ZOONOTIC INFECTIOUS DISEASES
Appropriations, 2022 ………………………………………………………………….. $693,272,000
Budget estimate, 2023 ………………………………………………………………… $703,272,000
Committee recommendation ……………………………………………………….. $793,772,000
The Committee recommendation for the activities of the National Center for Emerging and Zoonotic Diseases is $793,772,000, which includes $52,000,000 in transfers from the PPH Fund. The National Center for Emerging and Zoonotic Infectious Diseases aims to detect, prevent, and control infectious diseases from spreading, whether they are naturally occurring, unintentional, or the result of terrorism.
The Committee recommendation includes funding for the following activities in the following amounts:
Fiscal year 2022 Appropriation Committee recommendation
[In thousands of dollars] Budget activity
Antibiotic Resistance Initiative …………………………………………………………………………… 182,000 212,000
Vector-Borne Diseases ………………………………………………………………………………………. 54,603 69,603
Lyme Disease …………………………………………………………………………………………………… 20,500 26,000
Prion Disease …………………………………………………………………………………………………… 6,500 6,500
Chronic Fatigue Syndrome …………………………………………………………………………………. 5,400 5,400
CDC
p. 77
Lyme Disease.—The Committee recommendation provides an increase of $5,500,000 in recognition of the importance of prevention and control of Lyme disease and related tick-borne diseases, and encourages CDC to support surveillance and prevention of Lyme disease and other high consequence tick-borne diseases in endemic areas as well as areas not yet considered endemic. The Committee includes funding for CDC’s vector-borne diseases program to expand the programs authorized under the Kay Hagan Tick Act (Public Law 116–94) to promote a public health approach to combat rising cases of tick-borne diseases. The Committee directs CDC to develop and implement methods to improve surveillance to more accurately report the disease burden, including through the development of real time data for reporting Lyme disease and other tick-borne diseases, as well as a process for estimating the prevalence of Post-Treatment Lyme Disease Syndrome. The Committee directs CDC to direct funding to improve early diagnosis of Lyme and related tick-borne diseases to prevent the development of late stage disease and more serious and long-term disability. The Committee encourages CDC to coordinate with NIH, the National Institute of Mental Health [NIMH], and the National Institute of Neurological Disorders and Stroke [NINDS] on publishing reports that assess diagnostic advancements, methods for prevention, the state of treatment, and links between tick-borne disease and psychiatric illnesses. The Committee urges CDC, in coordination with NIH, to include in their surveillance the long-term effects on patients suffering from post-treatment Lyme disease syndrome, or ‘‘chronic 78 Lyme disease.’’ Additionally, given the impact of Lyme disease and the status of ongoing clinical trials, the Committee requests CDC provide a report to the Committees on Appropriations within 180 days of enactment on CDC’s research to date and recommendations on actions needed to facilitate a successful Lyme disease vaccine rollout that will build confidence and encourage uptake should a vaccine be approved by the FDA.
p. 79
Vector-Borne Diseases [VBDs].—The Committee is concerned about the risk of a vector-borne infectious disease outbreak in the U.S. and our readiness to quickly respond to and stop its spread. The recommendation includes an increase of $15,000,000 to support activities like those of the Regional Centers of Excellence program, including State-level surveillance and research being conducted by partners in order to prevent and rapidly respond to emerging VBDs across the United States. The Committee encourages CDC to examine options to provide greater coverage of the Northwest region for VBD resources. Additionally, the Committee recognizes the importance of strong surveillance data to monitor and forecast the risk of infectious disease outbreaks in the U.S. The Committee notes that the pandemic response necessitated the disruption of mosquito control and abatement efforts by many State and local health departments and notes the importance of continuing mosquito prevention efforts. The Committee is aware of the ongoing challenges faced in the Caribbean and the Pacific regarding control and management of VBDs, the increased risk for prevalence and spread of these diseases due to their tropical climate, and how cases in these islands can impact the rest of the country. The Committee urges CDC to support the training and research needs of the U.S. territories and encourages the use of the Mosquito Abatement for Safety and Health Program to provide grants and technical assistance to States and political subdivisions to prevent and control mosquito-borne diseases. In addition, the Committee requests CDC, in consultation with other appropriate agencies, to provide information in the fiscal year 2024 CJ on the ecological structure and epidemiological factors that must be known and monitored to estimate the mosquito-borne infectious disease outbreak risk.
NIH
P. 150
Lyme Disease and Related Tick-Borne Illnesses.—The Committee urges NIH to develop new tools that can more effectively prevent, diagnose, and treat Lyme disease, including its long-term effects, and other tick-borne diseases. The Committee encourages NIH to evaluate the effectiveness of laboratory tests associated with the detection of Borrelia burgdorferi to diagnose the disease early, which can improve the effectiveness of treatment. The Committee encourages the promotion and development of potential vaccine candidates for Lyme disease and other tick-borne diseases. The Committee urges NIH to conduct research to better understand modes of transmission for Lyme and other tick-borne diseases, including vertical transmission. The Committee encourages NIH to incentivize new investigators to enter the field of Lyme disease and other tick-borne disease research. The Committee directs NIH to coordinate with CDC on publishing reports that assess diagnostic advancements, methods for prevention, the state of treatment, and links between tick-borne disease and psychiatric illnesses.
DISCLOSURE OF CONGRESSIONALLY DIRECTED SPENDING ITEMS
p.367
Department of Health and Human Services. Health Resources and Services Administration, University of Maine System, ME, for rural health activities to prevent tick-borne diseases $1,653,000 Collins
Click here to learn more about the Center for Lyme Action.




















We invite you to comment on our Facebook page.
Visit LymeDisease.org Facebook Page