Galaxy Diagnostics launches new direct-detection test for Lyme disease
Press release from Galaxy Diagnostics, Inc.
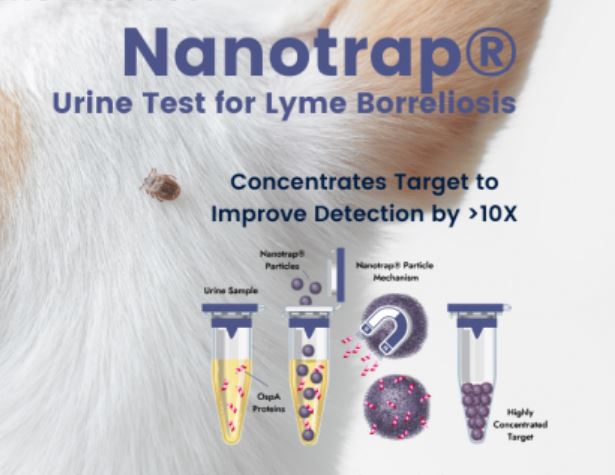
Galaxy Diagnostics, Inc., has announced the launch of the Nanotrap® Urine Test for Lyme Borreliosis.
This urine-based Lyme antigen test provides the most sensitive direct detection of Borrelia burgdorferi infection at all stages of the disease.
The test provides advantages antibody testing does not, namely:
- Identifies positive cases missed by CDC-recommended Two-Tiered Testing (TTT)
- Reduces concern for false positive results via direct detection of OspA proteins
- Uses easy-to-collect urine sample
The revolutionary test greatly increases the likelihood of Lyme disease confirmation via innovative Nanotrap® technology developed by Ceres Nanosciences. Nanotrap® particles capture and concentrate low abundance Outer surface protein A (OspA) in urine samples confirmed by a highly sensitive Western blot.
Confirming early-stage Lyme disease
Published data shows that the Nanotrap Urine Test is very effective for confirmation of early stage Lyme borreliosis in patients with EM rashes (24/24).
Galaxy validation data (unpublished) shows that the Nanotrap® Urine Test will often confirm active infection in patients with negative TTT results. Further research is needed to confirm clinical utility for other presentations of Lyme borreliosis, including Lyme arthritis, Lyme carditis, and neuroborreliosis.
“The addition of the Nanotrap® test aligns with our mission to bring the most scientifically advanced, most sensitive and most accurate sample enrichment testing to the forefront of flea and tick borne disease,” said Galaxy CEO Amanda Elam.
“Lyme disease is the fastest growing tick-borne illness in the United States. We are committed to improving the standard of care around detection of these elusive, underlying pathogens to catalyze improved clinical protocols for millions of patients globally.”
Galaxy advocates for a new standard of care in Lyme borreliosis testing and recommends a combination diagnostic protocol with Nanotrap® Urine test to confirm active infection and the CDC-recommended two-tiered testing to detect the presence of antibodies.
Click here for more information.
[Editor’s note: Kudos to the Bay Area Lyme Foundation. BALF funded Ceres Nanosciences to take its urine diagnostic for other diseases and adapt it for Borrelia. Then Ceres Nanosciences licensed it to Galaxy. We’re told that other tick-borne diseases are next.]




















We invite you to comment on our Facebook page.
Visit LymeDisease.org Facebook Page