Advanced imaging shows Lyme-related brain fog is real
By Nancy Dougherty
Johns Hopkins Medicine Lyme Disease Research Center
There are approximately 476,000 people diagnosed with Lyme disease annually in the US. Of these, an estimated 10-20% suffer from impairing symptoms well beyond the acute phase of infection.
Persistent symptoms include severe fatigue, pain, and cognitive problems. Vexingly, these symptoms can be difficult to validate using current diagnostic tests.
Patients are often told by their health practitioners: “your tests are normal, it’s in your head” (aka psychosomatic).
Researchers have discovered, however, that by using more advanced imaging modalities, brain changes can be objectively detected in Lyme disease patients.
For example, clinical researchers at Johns Hopkins University School of Medicine have used novel PET imaging, functional MRI (fMRI), and diffusion tensor imaging (DTI) to identify inflammatory, functional, and structural abnormalities in the brains of Lyme disease patients as compared to healthy controls.
Their findings indicate that Lyme-disease-associated brain alterations are biologic and measurable, not psychosomatic. These discoveries are significant but not yet well known by the physician community or broadly available to clinicians.
What does “brain fog” mean?
Brain fog is a term used to describe difficulties with cognitive functions such as working memory, focusing, concentrating, planning, organizing, word recall, processing speed, and mental fatigue.
Working memory enables one to do tasks quickly or easily solve a problem without looking up information. This includes quickly learning a new computer program or using names or numbers for a routine task.
Mental fatigue is akin to how one feels after taking a long test that requires remembering detailed information or doing a complicated mental task when short on sleep.
What are the limitations of current clinical tests for Lyme-associated brain fog?
Brain fog is commonly experienced by Lyme disease patients but difficult to detect because cognitive deficits can be too subtle for standard diagnostic tests to uncover.
Usual blood tests appear normal in Lyme encephalopathy or brain fog (CBC; CMP: kidney, liver, glucose, thyroid; ESR, CRP).
Serum blood testing for 2-tier IgG Borrelia burgdorferi antibody seropositivity may be negative in patients, such as those with past antibiotic treatment.
Clinically available MRI imaging is usually normal or shows nonspecific changes. Cerebrospinal fluid examination findings are usually normal as well. In some cases, the CSF fluid may show evidence of a mild form of encephalomyelitis, but this is rare.
Formal cognitive testing with a neuropsychologist to characterize the type and severity of cognitive problems can be a more helpful approach. Working memory and processing speed have been shown to be impacted in Lyme disease patients using standardized neuropsychological measures.[i] However, patient-reported cognitive complaints are subjective, and objective measures of cognitive decline are sought after by patients and clinicians.
What does more advanced neuroimaging reveal?
Non-standard research-grade neuroimaging technologies have discovered marked biologic abnormalities in patients with Lyme disease as compared to healthy controls.
A Columbia University brain PET imaging study (2009) found hypometabolism in Lyme-disease-associated encephalopathy.[ii]
A Johns Hopkins University School of Medicine brain PET imaging study (2018) revealed increased inflammation and glial activation in patients with Lyme-disease-associated persistent symptoms.[iii]
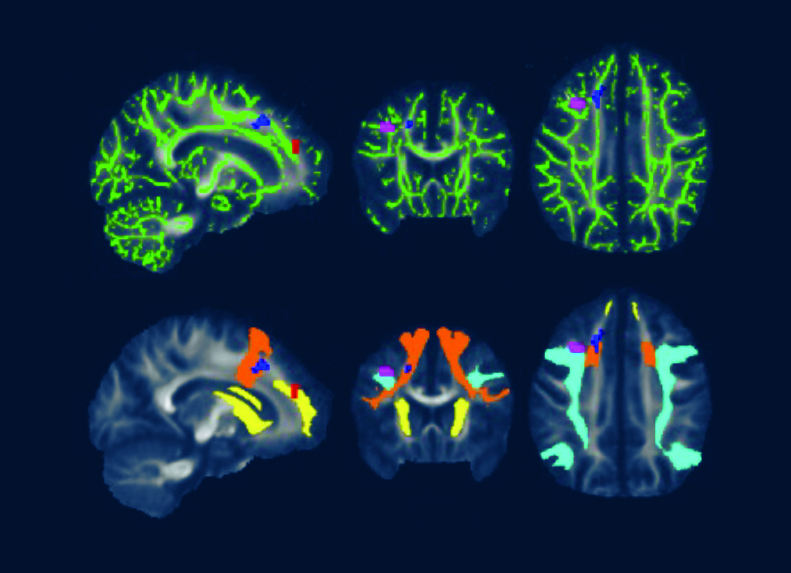
A 2022 Johns Hopkins University School of Medicine neuroimaging study utilized fMRI and DTI brain scans in conjunction with each other. These scans identified striking functional abnormalities as well as distinct structural changes in the white brain matter of Lyme disease patients.[iv]
What is the significance of these neuroimaging findings?
John Aucott, MD, Director of the Johns Hopkins Medicine Lyme Disease Research Center and Associate Professor of Medicine at Johns Hopkins University School of Medicine, explains, “The cause of Lyme-disease-associated persistent symptoms typically cannot be identified with regular MRIs, CT scans, or blood tests. However, in a research setting, more sophisticated PET, fMRI, and DTI imaging approaches have found significant objective abnormalities in the brains of Lyme disease patients compared with healthy controls.”
Cherie Marvel, PhD, lead author of the fMRI/DTI study and Associate Professor, Departments of Neurology & Psychiatry at Johns Hopkins University School of Medicine, describes fMRI as “a quantitative ‘brain stress test’ that measures brain function during cognitive tasks.”
Dr. Marvel explains that “the brain scans indicate Lyme disease patients’ brains work harder than normal and unexpectedly by activating white matter in the frontal lobe to try to maintain normal function.”
DTI, a measure of structural brain integrity, confirms abnormalities in the same white matter regions as observed in the fMRI scans.
These novel neuroimaging results provide new objective validation of a biologic basis for the brain fog reported by Lyme disease patients, including working memory impairment and slower processing speed.
The findings indicate Lyme-disease-associated brain fog is real and likely due to ongoing neuroinflammation driving brain dysfunction. More research is needed to better understand the diagnostic and therapeutic implications of these notable discoveries and to bring new insights and more advanced tools into the clinic to help patients.
Big picture
The Lyme-disease-associated advanced neuroimaging brain findings may be relevant to other infection-associated chronic illnesses where neuroinflammation is also significant, including long COVID and ME/CFS.
Nancy Dougherty is an Education and Communications Consultant for Johns Hopkins Medicine Lyme Disease Research Center. Follow her on Twitter: @NancyNDougherty.
References
[i] Touradji P, Aucott JN, Yang T, Rebman AW, Bechtold KT. Cognitive Decline in Post-treatment Lyme Disease Syndrome. Arch Clin Neuropsychol. 2019 Jun 1;34(4):455-465. doi: 10.1093/arclin/acy051. PMID: 29945190. https://pubmed.ncbi.nlm.nih.gov/29945190/
[ii] Fallon BA, Lipkin RB, Corbera KM, Yu S, Nobler MS, Keilp JG, Petkova E, Lisanby SH, Moeller JR, Slavov I, Van Heertum R, Mensh BD, Sackeim HA. Regional cerebral blood flow and metabolic rate in persistent Lyme encephalopathy. Arch Gen Psychiatry. 2009 May;66(5):554-63. doi: 10.1001/archgenpsychiatry.2009.29. PMID: 19414715. https://pubmed.ncbi.nlm.nih.gov/19414715/
[iii] Coughlin JM, Yang T, Rebman AW, Bechtold KT, Du Y, Mathews WB, Lesniak WG, Mihm EA, Frey SM, Marshall ES, Rosenthal HB, Reekie TA, Kassiou M, Dannals RF, Soloski MJ, Aucott JN, Pomper MG. Imaging glial activation in patients with post-treatment Lyme disease symptoms: a pilot study using [11C]DPA-713 PET. J Neuroinflammation. 2018 Dec 19;15(1):346. doi: 10.1186/s12974-018-1381-4. PMID: 30567544; PMCID: PMC6299943. https://pubmed.ncbi.nlm.nih.gov/30567544/
[iv] Marvel CL, Alm KH, Bhattacharya D, Rebman AW, Bakker A, Morgan OP, et al. (2022) A multimodal neuroimaging study of brain abnormalities and clinical correlates in post treatment Lyme disease. PLoS ONE 17(10): e0271425. https://doi.org/10.1371/journal.pone.0271425
Caption for featured photo: DTI brain images from a 2022 Johns Hopkins University School of Medicine study show white matter changes associated with post treatment Lyme disease.




















We invite you to comment on our Facebook page.
Visit LymeDisease.org Facebook Page