All about IgM–or why Lyme spirochetes are like stealth bombers
Borrelia burgdorferi, the bacteria that causes Lyme disease, can persist in animals and humans because it has evolved complex mechanisms to avoid the immune system.
In a 1996 interview with The Scientist magazine, Stephen Barthold, DVM, PhD, the researcher who developed the first mouse model of Lyme disease, described Borrelia burgdorferi’s ability to avoid immune detection as a form of cloaking. “It’s using some sort of stealth-bomber-type mechanism,” he said.
Since then, Professor Barthold has gone on to partner with many researchers in the pursuit of learning how B. burgdorferi causes chronic infection, including Monica Embers, PhD, from Tulane University.
Nicole Baumgarth, DVM, PhD, now the director of the Johns Hopkins Lyme and Tickborne Diseases Research and Education Institute, has years of experience collaborating with Barthold.
The latest research by Baumgarth, Barthold and others at University of California, Davis offers the science community one more clue as to how Borrelia is able to subvert the immune system leading to persistent infection in mice.
Their paper entitled, “Borrelia burgdorferi Infection–Induced Persistent IgM Secretion Controls Bacteremia, but Not Bacterial Dissemination or Tissue Burden,” has shed new light on how these pathogens persist in tissues, but present in very low numbers in the blood.
As Dr. Baumgarth tells me, “Blood is not the way Borrelia gets around the mice. Rather it migrates through tissues.”
These new findings may offer a possible explanation as to why disseminated Borrelia is both harder to treat, and so difficult to detect in blood samples.
Immune system basics
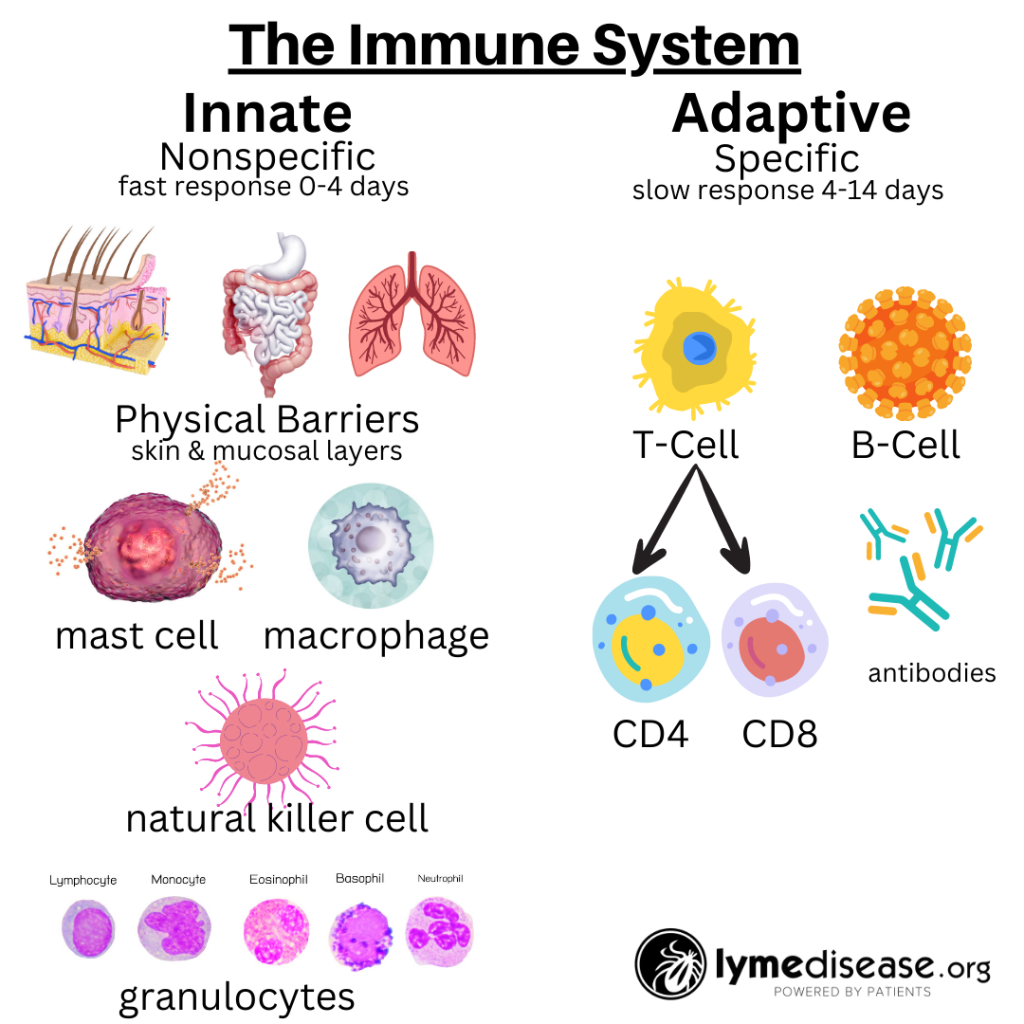
The immune system is roughly divided into two: the innate immune system and the adaptive immune system.
The innate immune system is our body’s first line of defense against pathogens and harmful substances. When working properly, it reacts immediately, but non-specifically, to all foreign invaders.
In contrast, the adaptive immune system is more targeted. It relies on prior exposure to learn and generate protective antibodies. The adaptive immune system remembers previous encounters and develops specific weapons (B-cells and T-cells) to fight each pathogen.
IgM vs IgG Antibodies
When the immune system detects any foreign substance, it produces antibodies which trigger the innate and later the adaptive immune system. IgM (immunoglobulin M) and IgG (immunoglobulin G) are two types of antibodies produced by the immune system.
IgM, the larger of these two immunoglobulins, is an early type of antibody to emerge in the development of an immune response. It acts as the initial defense against infections and is a strong activator of the complement system immune response that helps to clear pathogens.
The complement system consists of multiple proteins (C1 to C9) that summon phagocytes to the site of infection. Phagocytes (macrophages, neutrophils, lymphocytes) are components of the innate immune system that destroy pathogens. [For a crash course on the complement system watch this video.]
IgM is very effective in the blood stream. However, the size of IgM antibodies impedes their ability to penetrate all the tissues of the body. This limits IgM’s ability to send phagocytes into deeper tissues (eg. joints, heart, brain) where infection may be hiding.
IgG, a smaller, more penetrable antibody, is produced later in the immune response. IgG levels typically increase over a longer period of time, in some cases promoting the immune system to develop long-term immunity to future infection.
Study shows Borrelia impairs immune response
This new study shows how persistent Borrelia burgdorferi triggers a prolonged initial (IgM) immune response, and can impair a secondary (IgG) immune response.
This initial (IgM) response leads to fewer Borrelia burgdorferi (Bb) in the blood stream while the infection continues to spread throughout the body.
In addition, Borrelia’s prolonged IgM response in both humans and animals leads to a reduction in antibody-mediated clearance of the infection from deeper tissues.
The authors state, “Together the data demonstrated that IgG, but not IgM, is critical for the long-term control of B. burgdorferi tissue burden or disease induction. Despite that, Borrelia tissue dissemination in mice appeared very little affected by the rate of bacteremia, suggesting the B. burgdorferi main mode of dissemination in mice occurs by means other than via the blood.”
How IgM causes false-negatives
This research has shown that the standard definition of IgM as an acute response versus IgG as chronic response may be problematic in the classification of Lyme disease.
The Lyme disease Western Blot detects IgM and IgG responses to specific proteins found on Borrelia burgdorferi—for example: OspC (band 23-25), OspA (band 31), OspB (ban 34), BmpA protein (band 39), and flagellin protein (band 63-93).
In people with healthy immune systems, the Lyme disease IgM is normally detectable within a couple of weeks after infection, typically peaking around 4-6 weeks, then slowly declining over the next several months. The IgG begins around 4-6 weeks, peaking around 4-6 months, then slowly declining over the next several years.
Unfortunately, people who are immune-compromised, and/or fighting more than one infection (co-infection), may never develop a robust IgG response. The lack of the IgG response also prevents the immune system from finding and clearing bacteria embedded within deeper tissues, further impairing the healing process.
In the experimental Bb mouse model, despite extensive antibiotic treatment, IgM production continued for months. This is consistent with human Lyme disease studies demonstrating continued IgM response as long as 10 years, even in patients treated with antibiotics.
As the authors state, this is “a remarkable observation, given the short half-life of IgM, considered to be <24 hours.” Meaning a prolonged IgM is likely coming from the immune system reacting to persistent Borrelia.
The continued production of IgM in the blood stream may explain why Borrelia is so difficult to detect in blood samples.
And because the CDC discredits the presence of IgM after four weeks, the prolonged IgM is likely contributing to the high rate of false-negative standard tests for Lyme disease.
Per the CDC website, “the IgM Western Blot test result is only meaningful during the first four weeks of illness. If you have been infected for longer than 4 to 6 weeks and the IgG Western Blot is still negative, it is highly likely that the IgM result is incorrect (e.g., a false positive). This does not mean that you are not ill, but it does suggest that the cause of illness is something other than the Lyme disease bacterium.”
We now have evidence that this is simply not true in all cases.
Difficult to Detect
In 2019, I attended a vector-borne disease conference at University of California, San Francisco. While there, *Dr. Charles Chiu explained how his powerful direct detection DNA sequencing system—able to detect thousands of pathogens—was just not finding enough Borrelia burgdorferi in the blood stream of humans to work effectively. (See my live tweets of Chiu’s presentation here.)
I was just baffled how such a powerful tool could not consistently detect Lyme disease in humans. Now, knowing that IgM remains in the blood stream longer, keeping the bacteria numbers low, may help us understand this phenomenon.
The fact that the prolonged IgM reduces the presence of Bb in the blood stream helps to explain why next-generation serologic tests using direct detection of DNA or proteins may not be able to detect Bb in patients who are suffering from chronic Lyme disease.
[*Since then Dr. Chiu has gone on partner with Johns Hopkins University where they have developed a next-generation gene sequencing technique, called RNA-seq, to map the immune response to infection. And most recently Chiu has partnered with Columbia University to open the first West Coast Center for the Clinical Trials Network.]
Immune Disruption
Also covered in Baumgarth’s paper is another strategy Bb has developed to evade the immune system. Within 24 hours after the tick bite, Bb quickly invades and is detectible in the lymph nodes nearest the site of infection.
On the surface, this seems counterintuitive, as the lymph nodes contain many life-saving immune cells.
However, once Bb is in the lymph nodes, the spirochetes induce a signal that disrupts the “germinal centers” within the lymph node architecture. Ultimately, this alters the adaptive immune response of the lymphatics and impairs the immune system by limiting memory B and T cell production.
In addition, new research from Johns Hopkins has shown that Bb impairs dendritic cells. Dendritic cells are a type of immune cell spread throughout the body. Once activated, dendritic cells migrate to the lymph nodes, where they activate the adaptive immune response. Bb’s alteration of the dendritic cells also impairs the formation of memory B and T cells.
When the immune system functions properly, IgM is produced and triggers an immune response that is good at controlling bacteremia in the blood stream. From there, an IgG response is required to control dissemination outside the blood stream.
IgM and IgG also help to trigger macrophages, a type of white blood cell that envelops and destroys pathogens. The lack of this secondary IgG response prevents macrophages from getting the message to enter and clear Bb infection from deeper tissues—which contributes to persistent infection outside the bloodstream.
Conclusion
In conclusion, the authors “propose that the continued production of immune IgM is a manifestation of B. burgdorferi mediated B cell response subversion and represents an immune evasion strategy of B. burgdorferi. It may promote B. burgdorferi dissemination out of the blood and into the skin, where it can remain until attachment and bite of a tick will induce it to migrate toward the site of the tick bite.”
I was always told Bb likes to leave the blood stream and hide in zones of the body where it is protected from the immune system. This new study shows how Bb actually uses our own immune system to aide in its ability to hide. This strategy allows Bb to survive and attain its ultimate goal— which is not to kill the host, but to get picked up by another tick and spread to another host.
Over 40 years since the discovery of the spirochete responsible for Lyme disease, we are inching closer to understanding how Borrelia burgdorferi suppresses and evades the immune system.
LymeSci is written by Lonnie Marcum, a physical therapist and mother of a daughter with Lyme. She served two terms on a subcommittee of the federal Tick-Borne Disease Working Group. Follow her on Twitter: @LonnieRhea Email her at: lmarcum@lymedisease.org.
Reference
Hastey CJ, Olsen KJ, Elsner RA, Mundigl S, Tran GVV, Barthold SW, Baumgarth N. Borrelia burgdorferi Infection-Induced Persistent IgM Secretion Controls Bacteremia, but Not Bacterial Dissemination or Tissue Burden. J Immunol. 2023 Nov 15;211(10):1540-1549. doi: 10.4049/jimmunol.2300384. PMID: 37782044.




















We invite you to comment on our Facebook page.
Visit LymeDisease.org Facebook Page