Why Lyme disease treatment sometimes doesn’t work
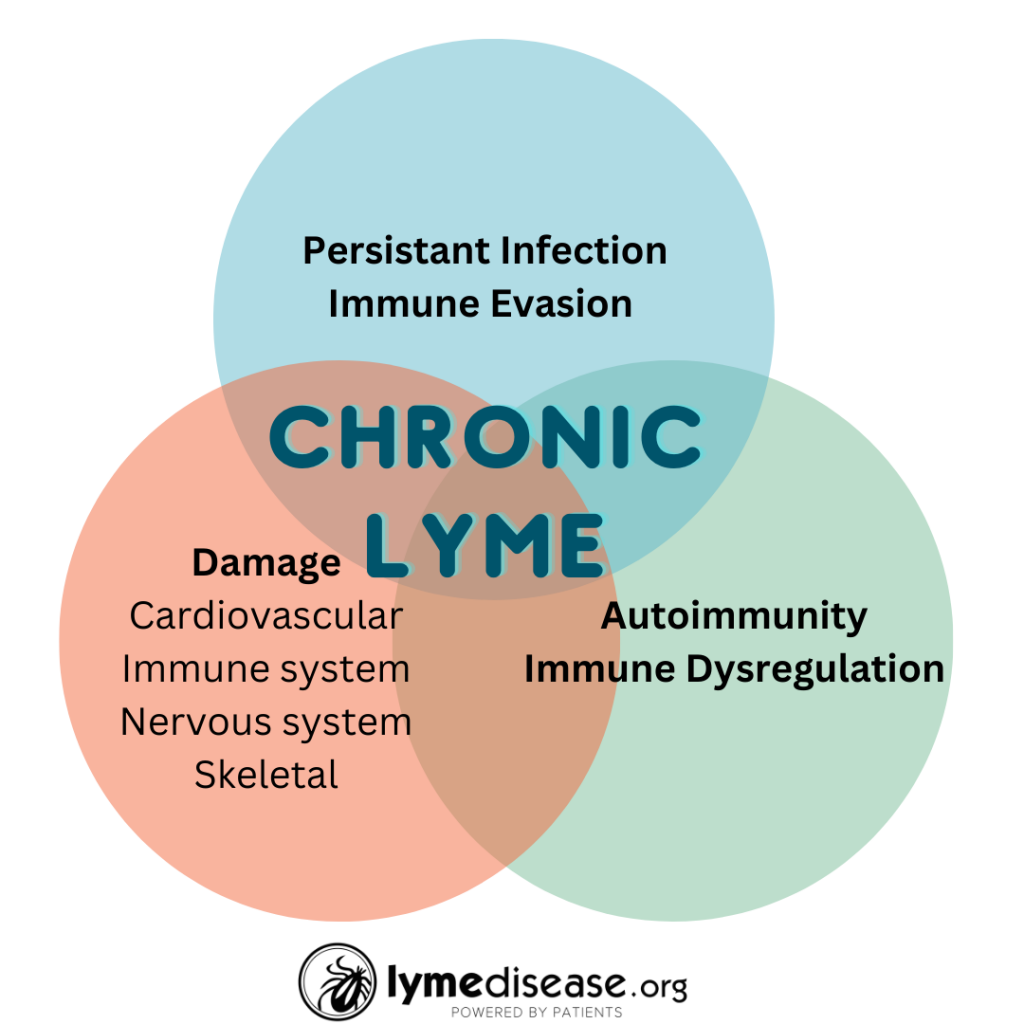
It is well known that anywhere from 10-30% of patients treated for early Lyme disease will be left with persistent symptoms, sometimes lasting months or even years. Of those who are diagnosed later, even more will suffer persistent symptoms.
There are several potential mechanisms for persistence. These include delayed treatment, persistent infection, co-infections, re-activated infections, genetics, autoimmunity and physical damage to the body caused by the infection. Regardless of the cause, these chronic symptoms share a common element—antibiotic treatment failure.
A recent paper by Heather Adkinson and Monica Embers from Tulane University takes a deep dive into three notable mechanisms of treatment failure. It’s entitled Lyme disease and the pursuit of a clinical cure, published in Frontiers in Medicine.
The three factors they examine are: (1) autoimmunity, (2) post-infectious immune-mediated sequelae [aftereffects], and (3) persistent infection.
Autoimmunity
The term autoimmunity describes a condition when our immune system gets confused. Rather than protecting us from foreign germs, it starts attacking our own body’s healthy cells and tissues. Common conditions associated with autoimmunity are type 1 diabetes, lupus, and rheumatoid arthritis.
Lyme arthritis happens when either active infection or remnants of Borrelia cause the immune system to attack the tissues inside of our joints. Some refer to this auto-inflammatory condition as a form of autoimmunity.
Research has shown that patients with persistent symptoms following treatment for Lyme disease have higher levels of antibodies against proteins found in the nervous system—something that is also seen in patients with multiple sclerosis. This finding also supports the hypothesis of an autoimmune component to chronic neurological Lyme disease.
Post-infectious immune-mediated sequelae
The Borrelia burgdorferi spirochete itself does not directly damage human or animal tissues. Instead, the signs and symptoms of Lyme disease in humans are caused by the immune system’s response to the bacteria.
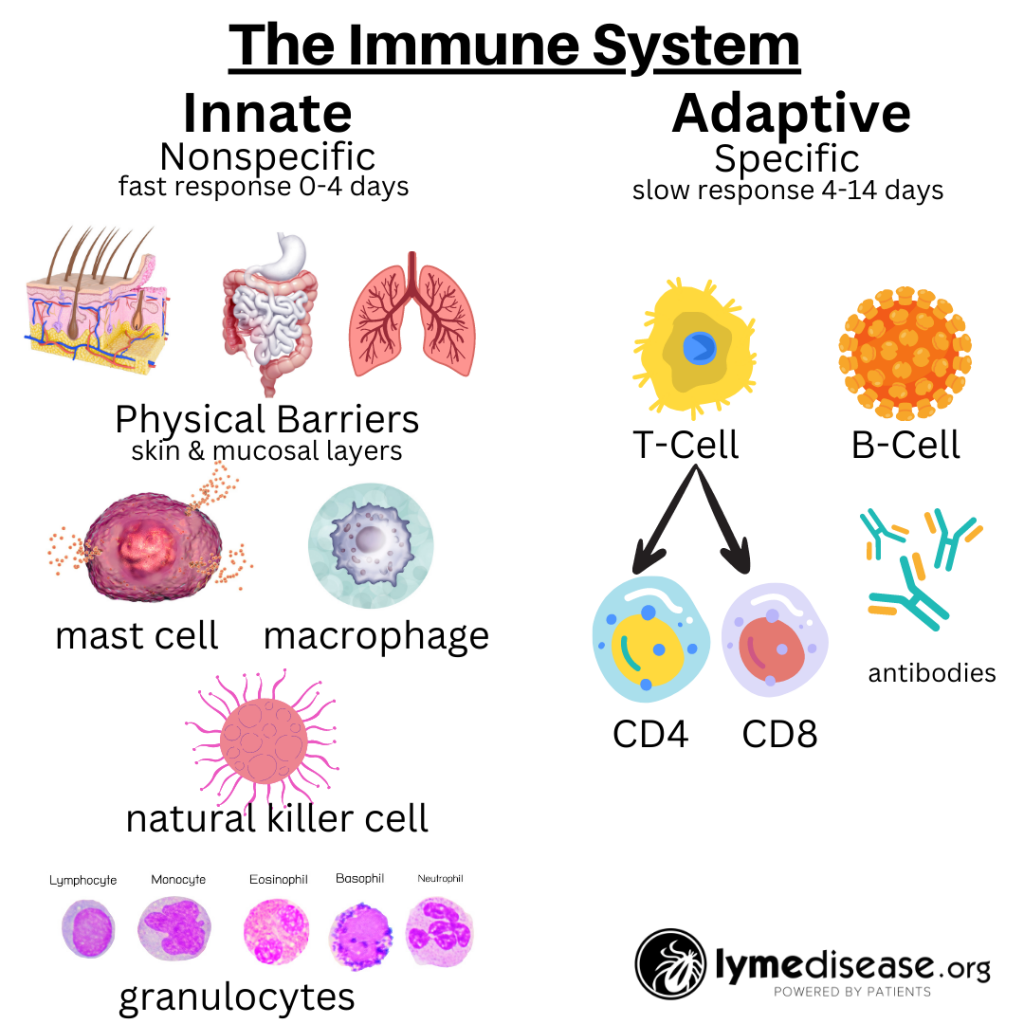
The immune system is roughly divided into two: the innate immune system and the adaptive immune system.
The innate immune system—the one we’re born with—is our body’s first line of defense against pathogens and harmful substances. When working properly, it reacts immediately, but non-specifically, to all foreign invaders.
In contrast, the adaptive immune system is more targeted. It relies on previous exposure to learn and generate specific antibodies. It remembers previous encounters and develops specific weapons (B-cells and T-cells) to fight each pathogen.
The adaptive immune system makes copies of these weapons so that the next time they can react faster and more efficiently. This part of our immune system is continuously adapting to our exposures and typically gets stronger with each encounter.
Unfortunately, even with a healthy immune system, we can become reinfected with Lyme and other tick-borne diseases, repeatedly. Thus, we become sick every time we are infected. When Borrelia burgdorferi infects the human body there is a complex interaction between various pro-inflammatory and anti-inflammatory elements of our immune system.
Ideally, with early treatment, our bodies are able to eliminate all traces of the bacteria. However, if the infection goes untreated or if remnants of the bacteria remain, this can cause a cascade of pro-inflammatory chemokines and cytokines. These inflammatory elements can damage our tissues leading to an assortment of symptoms involving any or all systems of the body including the skin, joints, heart, gut, brain and more.
Mast cell activation syndrome
Mast cells are the oldest and most primitive element of our innate immune system. I’ve written extensively about what happens when mast cells go haywire leading to mast cell activation syndrome—something recognized more and more in patients with chronic Lyme.
The authors also cite several studies where it appears that “interferon gamma (IFN-ɣ) is a major instigator in the inflammatory pathology seen in early LD, especially in the joints and nervous system.” This suggests the benefit of monitoring inflammatory markers and taking measures to reduce inflammation during and after treatment for Lyme disease. For instance running a cytokine panel when running blood work, sticking to a low inflammatory diet, taking NSAIDS as directed or other methods for reducing the Herxheimer reaction including many natural remedies for reducing inflammation.
The Johns Hopkins University SLICE study has also found several markers that predict which acutely ill patients will develop post-treatment Lyme disease syndrome (PTLDS.) Specifically, they found that the T-cell chemokine known as CCL19 remains elevated in the patients who do not improve following standard treatment for Lyme disease.
Persistent infection
Borrelia spirochetes have developed incredible stealthy tactics that allow it to evade the immune system and persist for years in their animal or human host. Left untreated, Borrelia establishes a chronic and persistent infection—this fact is not controversial.
Where the controversy lies is in whether Borrelia infection can persist after treatment with antibiotics and if those persister cells are what is causing the symptoms of PTLDS.
Dr. Ying Zhang, of Johns Hopkins University, has done a great deal of research on antibiotic resistant infections. He likens persister cells to weeds growing in a lawn. The antibiotics, like the lawnmower, knock out the growing form of the spirochete but not the persister forms, like the root of a dandelion. Later, just like the weeds, the bacteria will grow back.
The problem with these persister Borrelia cells is they are not easy to grow by culture in the laboratory—a laboratory technique that is used to show if an infection is present in a patients’ blood sample.
Without proof of viable bacteria by culture, it’s hard to prove what causes persistent symptoms. Nonetheless, we know that non-viable Borrelia remnants are capable of generating a disease-causing inflammatory response.
Delayed treatment
Another complicating factor is delayed diagnosis and treatment. Many studies have shown that patients who receive antibiotic treatment within four weeks of initial infection are most likely to have successful outcomes.
Unfortunately, many patients don’t receive treatment during that early window of opportunity. In a MyLymeData study, only 7% of patients were diagnosed within the first month. Nearly 80% reported that it took more than six months for them to receive a correct diagnosis.
Delayed diagnosis is partly due to the unreliability of standard Lyme tests, which measure antibodies to Lyme, not the infection itself. Because it can take the human body four to six weeks to develop detectable antibodies, these tests result in many false negatives. This is why an early diagnosis of Lyme disease should be based on symptoms, physical findings (e.g., rash), and the possibility of exposure to infected ticks.
As the authors point out, a delayed diagnosis of Lyme leads to late-stage disease, which is much more difficult to treat. Moreover, the standard treatment for Lyme disease uses doxycycline—a bacteriostatic antibiotic.
Bacteriostatic antibiotics merely inhibit the ability of the bacteria to grow, relying on the immune system to kill and remove the bacteria. If someone is immune compromised, which can happen when fighting multiple co-infections, their immune system will have a harder time clearing the infection.
Next Generation Treatments
As described above, there may be several reasons for persistent symptoms following treatment for Lyme disease. All have one thing in common: the standard treatment for Lyme disease fails a significant number of patients each year.
Using the CDC’s estimate, let’s assume 476,000 new cases of Lyme disease per year. If 20% remain ill, that leaves nearly 100,000 patients per year with chronic symptoms following standard treatment. This warrants further study, new clinical trials, and improved treatment options.
Treatment options for eradicating the infection may include antibiotics used alone or in combination with other drugs or even essential oils. Drugs that are being considered include disulfiram, vancomycin, hygromycin A, daptomycin, artemisinin, cefoperazone, azlocillin, cefotaxime and more.
Because a good portion of the initial and continuing symptoms of Lyme are generated by the immune response, additional options for therapy may include immune-modulating treatments. While there are a few approved monoclonal antibodies for treating bacterial infections, none are currently approved for the treatment of PTLDS.
In Pursuit of a Cure
Recently, I witnessed something I’d only dreamt about—scientists, researchers, and patient representatives from many different infection-associated chronic illnesses together in one room.
In June 2023, The National Academies of Sciences, Engineering, and Medicine (NASEM) held a two-day event in Washington DC titled Toward a Common Research Agenda in Infection-Associated Chronic Illnesses: A Workshop to Examine Common, Overlapping Clinical and Biological Factors.
In the end, I think those of us who watched the presentations came away with a feeling of validation about the illnesses we represent and a huge boost in knowledge of the similarities of others suffering from long-haul diseases. I’ve heard from several participants who are looking forward to the collaborative relationships that were built through NASEM continuing in the future. (SEE ALSO: “Words matter”: A new way of thinking about long-haul diseases.)
Like the authors of this paper and those at NASEM, I am hopeful that with advancements in personalized medicine, and collaborations with other fields allowing for scientific breakthroughs, we will find a cure for Lyme disease, making persistent Lyme disease and other infection-associated chronic conditions a thing of the past.
LymeSci is written by Lonnie Marcum, a Licensed Physical Therapist and mother of a daughter with Lyme. She served two terms on a subcommittee of the federal Tick-Borne Disease Working Group. Follow her on Twitter: @LonnieRhea Email her at: lmarcum@lymedisease.org.
Reference
Adkison H, Embers ME. Lyme disease and the pursuit of a clinical cure. Front Med (Lausanne). 2023 May 24;10:1183344. doi: 10.3389/fmed.2023.1183344. PMID: 37293310; PMCID: PMC10244525.




















We invite you to comment on our Facebook page.
Visit LymeDisease.org Facebook Page