LYMEPOLICYWONK: Lyme disease testing—the CDC, LabCorp and stories that don’t add up
Dr. Paul Mead of the Centers for Diseases Control and Prevention (CDC) recently stated in the Wall Street Journal that the CDC’s recommended two-step process (which requires a positive ELISA test before a Western blot can be given) is accurate and was developed specifically to aid diagnosis of Lyme disease. A stunning assertion given that it directly conflicts with Dr. Mead’s previous testimony before the Connecticut Department of Public Health and the Attorney General’s office. It is also at odds with the CDC case definition for Lyme disease, which recognizes the validity of a stand-alone Western blot. This turnaround, coupled with LabCorp’s recent decision to abandon a stand-alone Western blot, has patients up in arms. And, rightly so.
Over the years, Lyme advocacy organizations have raised concerns to the CDC that doctors were relying on the CDC surveillance case definition to diagnose Lyme disease and, consequently, were missing far too many cases. Whenever we asked the CDC to change this definition, we were told two things by CDC officials. One – the surveillance definition was never intended for diagnosis. Two – they could not change the surveillance definition even if they wanted to since it was determined by an independent third party over whom the CDC has no control, the Council for State and Territorial Epidemiologists (CSTE). Dr. Mead himself spoke eloquently about the difference between Lyme surveillance and diagnosis at the hearing of the Connecticut Attorney General in 2004:
“A clinical diagnosis is made for the purpose of treating an individual patient and should consider the many details associated with that patient’s illness.Surveillance case definitions are created for the purpose of standardization, not patient care; they exist so that health officials can reasonably compare the number and distribution of “cases” over space and time. Whereas physicians appropriately err on the side of over-diagnosis, thereby assuring they don’t miss a case, surveillance case definitions appropriately err on the side of specificity, thereby assuring that they do not inadvertently capture illnesses due to other conditions….
No surveillance case definition is 100% accurate. There will always be some patients with Lyme disease whose illness does not meet the national surveillance case definition. For this reason, CDC has stated repeatedly that the surveillance case definition is not a substitute for sound clinical judgment. Given other compelling evidence, a physician may choose to treat a patient for Lyme disease when their condition does not meet the case definition.”
In fact, at the beginning of the surveillance case definition, the CDC notes that the “surveillance case definition was developed for national reporting of Lyme disease; it is not intended to be used in clinical diagnosis.”
I suppose Dr. Mead could choose to split hairs by claiming surveillance is not the same as diagnostic tests. If so, this would be the first time the CDC has split this particular hair. It is also odd because in 2008 and after years of complaints by patients about the misuse of Lyme disease surveillance case definitions for diagnosis, the CSTE revised the case definition so that stand-alone IgG Western blot tests would meet surveillance criteria. Both are now characterized as “qualified laboratory assays.”
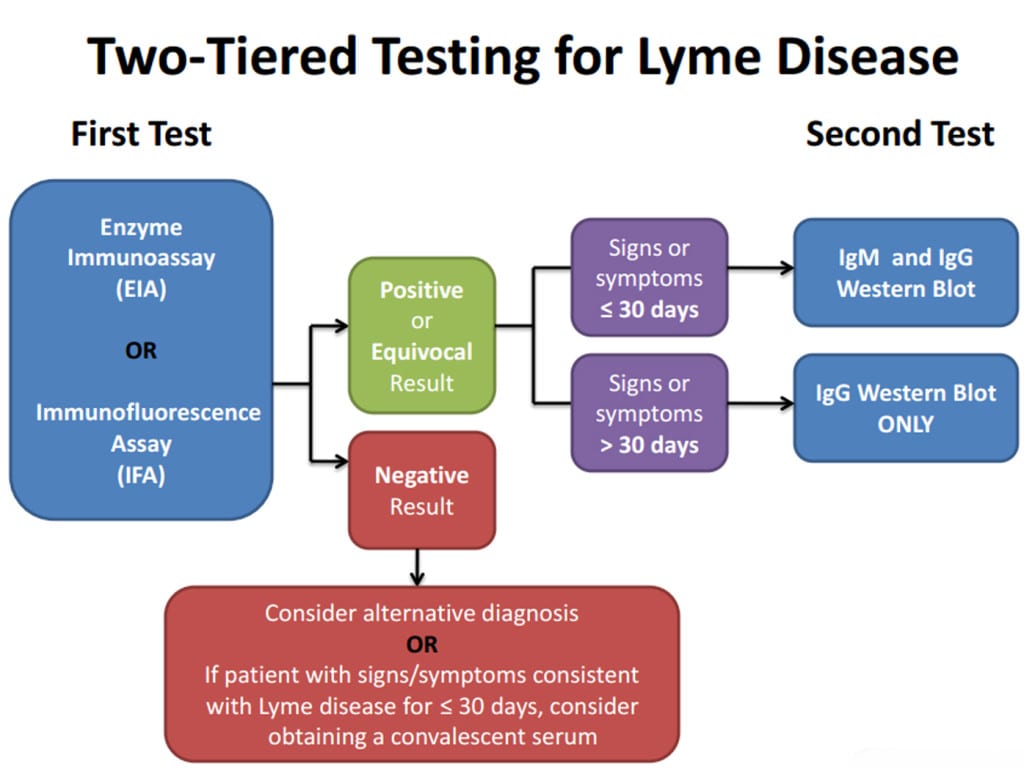
Laboratory Criteria for Diagnosis
For the purposes of surveillance, the definition of a qualified laboratory assay is
- Positive Culture for B. burgdorferi, OR
- Two-tier testing interpreted using established criteria1, where:
- Positive IgM is sufficient only when ≤30 days from symptom onset
- Positive IgG is sufficient at any point during illness
- Single-tier IgG immunoblot seropositivity using established criteria.1-4
- CSF antibody positive for B. burgdorferi by Enzyme Immunoassay (EIA) or Immunofluorescence Assay (IFA), when the titer is higher than it was in serum.
So why the indignant back peddling? This could be as amusing as an episode of Jon Stewart, where he reminds public figures that their actions are recorded on film or in public hearings or even in their own case definition. So funny—except we are talking about patients’ lives.
I wish Dr. Mead would reread his interview with NPR in 2013, where he claims that the CDC recognizes chronic Lyme disease as a real problem:
“The question is whether it’s due to persistent infection or some immunologic effect, and what’s the best way of treating it . . . It confirms what we’ve thought for a long time: This is a large problem. The bottom line is that by defining how big the problem is we make it easier for everyone to figure out what kind of resources we have to use to address it.”
Yeah, about those resources … in their July 2014 newsletter LabCorp announced they will no longer run standalone Western blots. The rumor is the change came about after strong-arm tactics by the CDC. Is it only a rumor? Lyme disease is a serious threat to public health, and making it harder to diagnose makes the problem worse. Let’s direct our efforts toward prompt, correct diagnosis and appropriate treatment. The CDC’s blatant rewrite of history only serves to increase public distrust of a government agency that is supposed to be transparent, respectful, and serve the taxpayers they supposedly work for.
The LYME POLICY WONK blog is written by Lorraine Johnson, JD, MBA, who is the Chief Executive Officer of LymeDisease.org, formerly CALDA. Contact her at lbjohnson@lymedisease.org. On Twitter, follow me @lymepolicywonk




















After taking the more sensitive IGenex test, I’m positive for Lyme and babesia. Even after taking amoxicillan for 10 days after finding the tick embedded in my back, I still contracted this bacteria and parasitic infection. The current IDSA and CDC guidelines would have guaranteed my continued illnesses. To force doctors to become co-conspirators is unethical, shameful and deplorable. It’s time for the federal government to take responsibility for what they have done and insurance company lobbyists to butt out.
Brava, Lorraine. This is a fabulous analysis of these pervasive issues with LD testing.
I have spoken with a scientist who was present at the Dearborn conference and he confirmed that many of the scientists present at the conference expressed their concerns that while the two-tier test was very specific, the test lacked the sensitivity to be an appropriate diagnostic assay. These scientists only agreed to support the Dearborn criteria if it was only to be used solely for surveillance purposes. It has been recorded on video, that Dr. Paul Lantos and Dr. Paul Auwaerter approached you at the most recent IDSA protest to confirm that even the IDSA agrees with patient advocates that the current recommended tests for Lyme disease are terrible.
So Dr. Mead’s and the CDC’s recent turn around is obviously not based on any scientific consensus, but politics. The question is, whose politics and why? Who stands to benefit? Since it is not Lyme patients, why does the well-being of Lyme patients matter so little to the CDC?
Fortunately, there is a way for patients who have been harmed by the F.D.A. approved two-tier tests to take action. These harms can include delayed diagnosis, misdiagnose, and improper or no treatment.
Medwatch is the branch of the U.S. Food and Drug Administration (FDA) that receives consumer complaints regarding medications and medical devices. If Medwatch receives enough complaints, the FDA will be forced to investigate this issue.
Use this MEDWATCH link to file your report:
https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=consumer.reporting1
Or contact the FDA Consumer Complaint Coordinator for the state in which you reside:
http://www.fda.gov/Safety/ReportaProblem/ConsumerComplaintCoordinators/default.htm