LYME SCI: Can treating mast cells help neurological Lyme disease?

One night years ago, when my daughter was at her sickest, she asked me, “Is the chandelier hanging sideways?” With a curious look on my face, I answered, “No. Why?”
She said everything in the room, including the walls, looked tilted. Like in Whoville, the town in Dr. Seuss’s book “Horton Hears a Who!”
A month later, a prestigious children’s hospital diagnosed her with myalgic encelphalomyelitis (ME) and chronic fatigue syndrome (CFS). The doctors gave no other explanation for her laundry list of symptoms, including a recurring fever.
Little did we know that she had a raging infection inside her brain. Her nightly visits to Whoville continued for months. They finally stopped, after she was diagnosed with neurological Lyme disease and co-infections, and treated with antibiotics.
Patients with late-stage Lyme are no strangers to the neurological manifestations of this illness. Symptoms like brain fog, anxiety, depression and insomnia are common complaints in this population. But what causes all these symptoms? Inflammation. Like in many other illnesses, inflammation is the enemy.
At ILADS 2019, Dr. Tania Dempsey emphasized the importance of diagnosing and treating mast cell activation syndrome when it is suspected in the course of Lyme disease.
Mast cells are a complicated aspect of our immune system. When mast cells go haywire, they cause inflammation. (I’ve written extensively about mast cell diagnosis, treatment and how it helped solve the puzzle of my daughters illness.)
Dr. Dempsey has found that incorporating mast cell treatment into her Lyme protocols helps to reduce many of the symptoms of “Lyme brain” by controlling inflammation. In my daughter’s case, finding the right mast cell protocol was a critical step in getting her into remission.
Disseminated neurological Lyme disease
Neurological Lyme disease symptoms are largely invisible and difficult to quantify. Thus, many physicians write them off as psychosomatic, leaving these patients with no validation or treatment options.
Until recently, there hasn’t been a good measurement for patients with these long-standing neurological symptoms following treatment for Lyme disease.
Two separate studies published in 2019 by renowned researchers—one from Johns Hopkins University, the other from Harvard—have finally given us quantifiable, physiological reasons for these debilitating neurological symptoms.
The Johns Hopkins study revealed chemical changes and widespread inflammation in the brains of patients with persistent symptoms following treatment for Lyme disease. The Harvard study showed nerve damage (small fiber neuropathy) and decreased blood flow in the brains that may also contribute to dysautonomia. (Dysautonomia is a malfunction of the autonomic nervous system. It can cause a variety of troublesome symptoms.)
Left untreated, Lyme disease can spread to the nervous system. There are a multitude of diseases associated with neurologic Lyme:
- Lyme meningitis – inflammation of the membranes that cover the brain/spinal cord.
- Lyme encephalitis – inflammation inside the brain
- Lyme myelopathy – inflammation of the spinal cord
- Lyme cranial neuritis – inflammation of the cranial nerves
- Lyme neuropathy – inflammation of the peripheral nerves
- Bannwarth’s syndrome – “terrible triad” of meningitis, cranial neuritis and painful neuropathy
Nervous system basics
The nervous system has two main parts. The central nervous system consists of the brain and spinal cord. The peripheral nervous system includes all the nerves outside of the central nervous system. Lyme disease can infect either or both.
The central nervous system has a special protective layer called the blood brain barrier (BBB). It lets in nutrients like oxygen and glucose, and keeps out pathogens. Without the BBB, the brain would be open to infection from every germ that enters the blood stream.
A new study from UC Berkeley has found that a leaky BBB leads to memory loss and cognitive dysfunction in older mice. By giving these rodents drugs to reduce inflammation in their brains, the researchers reversed their signs of aging.
“When you remove that inflammatory fog, within days the aged brain acts like a young brain. It is a really, really optimistic finding, in terms of the capacity for plasticity that exists in the brain. We can reverse brain aging,” said one of the researchers, Professor Daniela Kaufer. Their study has profound implications for human brains too.
Mast cells: a gateway to the brain and nervous system
Mast cells play a major role in defending our bodies from pathogens. On the brain side of the blood-brain barrier, they serve as “universal alarm cells” that start the inflammatory cascade.
During infection of the central nervous system, the number of mast cells will increase, contributing to inflammation. During chronic inflammation, the BBB becomes more permeable—leaky– allowing mast cells to travel out of the brain. Unfortunately, the leaky BBB also allows large white blood cells and blood-borne pathogens into the brain.
Mast cells contain many different types of chemicals. The most abundant one is tryptase, a powerful enzyme released during mast cell activity. At ILADS, Dr. Theo Theoharides explained that tryptase acts almost like a “meat tenderizer.” As you can imagine, something that can “tenderize” the nervous system would be pretty destructive.
When mast cells become over-reactive, they create a continuous loop of inflammation that adversely impacts nerves and the nervous system.
In my daughter’s case, the mast cells became activated during her untreated infection. They remained so until we removed triggers, incorporated a low-histamine diet, and added treatment for mast cell activation syndrome (MCAS). After two years of a strict diet and MCAS treatment, it appears her mast cells have partially returned to normal.
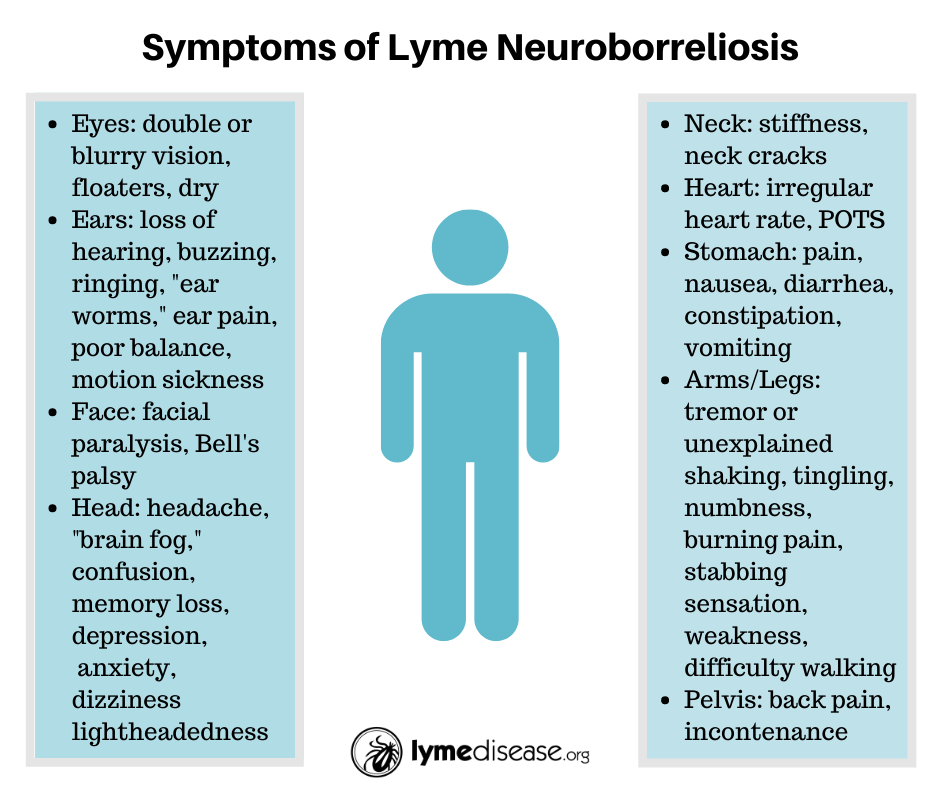
Symptoms of neurological Lyme disease
The MyLymeData project asked Lyme patients who were still ill to identify their three worst symptoms. It turns out, the most frequently reported “worst symptoms” were neurological.
The most frequently reported neurological Lyme disease symptoms in MyLymeData included memory loss and cognitive impairment, sleep impairment, psychiatric manifestations, headaches, and neuropathy.
Infections within the CNS are much more difficult to treat, because most oral antibiotics do not cross the BBB. Even with IV ceftriaxone, the drug of choice for neurologic Lyme disease, many patients will be left with chronic symptoms.
While doctors don’t all agree about the cause of these ongoing symptoms, many ILADS doctors believe treatment should continue until symptoms have resolved. Some also report that intravenous immunoglobulin (IVIG) or plasmapheresis can also be helpful in treating infection-induced autoimmune encephalitis.
In addition, as we learned at ILADS this year, many doctors are seeing improved results by incorporating MCAS treatment into their Lyme protocols.
There is hope
As my daughter has shared, you can heal from Lyme and mast cell activation. Jesse Colin Young, Kris Kristofferson, Amy Tan, Yolanda Foster, and many others have reported that they got their brains back after being treated for Lyme disease.
As science sheds new light on disorders of the nervous system, the Lyme community stands to gain. My message to you is, don’t give up hope.
LymeSci is written by Lonnie Marcum, a Licensed Physical Therapist and mother of a daughter with Lyme. Follow her on Twitter: @LonnieRhea Email her at: lmarcum@lymedisease.org .
References:
Afrin LB, et al. (2015) Mast cell activation disease: An underappreciated cause of neurologic and psychiatric symptoms and diseases. Brain, Behavior, and Immunity, 50:314-321. DOI: https://doi.org/10.1016/j.bbi.2015.07.002
CDC | Lyme Disease | Accessed 1/2/2020 https://www.cdc.gov/lyme/index.html
Dattwyler, R. J, et al. (1988). Treatment of late Lyme borreliosis–randomised comparison of ceftriaxone and penicillin. Lancet, 1(8596), 1191-1194.
Edis AJ, Shepherd JT. (1970) Autonomic Control of the Peripheral Vascular System. Arch Intern Med. 1970;125(4):716–724. doi:https://doi.org/10.1001/archinte.1970.00310040140020
Erol AYG (2015) The Role of Mast Cells and Neuroglia in Neuroinfectious Diseases. J Neuroinfect Dis 6:190. doi:10.4172/2314-7326.1000190
Fallon, B. A., et. al. (2008). A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology, 70(13), 992-1003. doi:10.1212/01.WNL.0000284604.61160.2d
History of Blood-Brain Barrier. The Davis Lab, University of Arizona. Accessed 12/14/19 https://web.archive.org/web/20120425131005/http://davislab.med.arizona.edu/content/history-blood-brain-barrier
Johnson L, Shapiro M, Mankoff J. (2018) Removing the Mask of Average Treatment Effects in Chronic Lyme Disease Research Using Big Data and Subgroup Analysis. Healthcare (Basel). Oct 12;6(4). pii: E124. doi: 10.3390/healthcare6040124.
Johnson L, Wilcox S, Mankoff J, Stricker RB. (2014) Severity of chronic Lyme disease compared to other chronic conditions: a quality of life survey. PeerJ. 2014; 2: e322 doi: 10.7717/peerj.322
Kao, W.A., et al. (2017) Identification of Tp0751 (Pallilysin) as a Treponema pallidum Vascular Adhesin by Heterologous Expression in the Lyme disease Spirochete. Sci Rep 7, 1538 doi:10.1038/s41598-017-01589-4
Köhler-Forsberg O, et al. (2019) A nationwide study in Denmark of the association between treated infections and the subsequent risk of treated mental disorders in children and adolescents. JAMA Psychiatry. 1;76(3):271-279. doi: 10.1001/jamapsychiatry.2018.3428.
Krawczuk, K., et al. (2020) Comparison of Neuroborreliosis Between Children and Adults. The Pediatric Infectious Disease Journal: Vol 39 – 1; p 7–11 doi: 10.1097/INF.0000000000002493]
Liegner, K. B., et al. (1997). Lyme disease and the clinical spectrum of antibiotic responsive chronic meningoencephalomyelitides. Journal of Spirochetal and Tick-borne Diseases, 4, 61-73.
Logigian, E. L., Kaplan, R. F., & Steere, A. C. (1999). Successful treatment of Lyme encephalopathy with intravenous ceftriaxone. J Infect Dis, 180(2), 377-383. doi:10.1086/314860
Logigian, E. L., et al. (1997). Reversible cerebral hypoperfusion in Lyme encephalopathy. Neurology, 49(6), 1661-1670. DOI: https://doi.org/10.1212/WNL.49.6.1661
Nocton, J.J.; et al. (1996) Detection of Borrelia burgdorferi DNA by polymerase chain reaction in cerebrospinal fluid in Lyme neuroborreliosis. J. Infect. Dis. 174, 623–627. DOI: 10.1093/infdis/174.3.623
Skaper SD, Giusti P, Facci L. (2012) Microglia and mast cells: two tracks on the road to neuroinflammation | The FASEB Journal DOI: https://doi.org/10.1096/fj.11-197194
Theoharadis, TC. (1990) Mast cells: The immune gate to the brain. Life Sciences, 46: 607-617; DOI: https://doi.org/10.1016/0024-3205(90)90129-F
Theoharides TC, Valent P, Akin C. (2015) Mast Cells, Mastocytosis, and Related Disorders. N Engl J Med. 2015 Jul 9;373(2):163-72. doi: 10.1056/NEJMra1409760.
Tran H, et al. (2019) Mast Cells Induce Blood Brain Barrier Damage in SCD by Causing Endoplasmic Reticulum Stress in the Endothelium. Front. Cell. Neurosci. 13:56. doi: 10.3389/fncel.2019.00056
Valent P, et al. (2012) Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 157(3):215-25. doi: 10.1159/000328760.
VanElzakker MB, Brumfield SA and Lara Mejia PS (2019) Neuroinflammation and Cytokines in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Critical Review of Research Methods. Front. Neurol. 9:1033. doi: 10.3389/fneur.2018.01033
Verma, V., et al. (2014). A case of chronic progressive lyme encephalitis as a manifestation of late lyme neuroborreliosis. Infectious disease reports, 6(4), 5496. doi:10.4081/idr.2014.5496




















We invite you to comment on our Facebook page.
Visit LymeDisease.org Facebook Page