O n April 28, I was honored to give a keynote speech at the LymeX Innovation Accelerator, which was sponsored by the US Department of Health and Human Services and the Cohen Foundation. The Cohen Foundation has funded more research for Lyme disease than any other private organization. I spoke about why we need patient-partnered research to ensure that studies address the needs of patients living with the disease. I also talked about the role of the MyLymeData registry and the need to protect patient data so that it is used to benefit patients.
You can watch my talk in the membership area.
Transcript of keynote speech:
LymeDisease.org is one of the oldest Lyme advocacy organizations in the nation — over thirty years old. It is also the largest and most trusted Lyme disease patient communications network. Our mission is to harness the power of tens of thousands of patients to improve patient care and accelerate the pace of Lyme disease research. We do this through communications, the MyLymeData patient registry and research platform, and science-based advocacy.
Today I am going to talk with you about what we need to do as a community to accelerate the pace of research and why I believe involving patients as research partners is essential to develop community trust and to ensure that research addresses questions that are important to patients and uses outcomes that patients value. Let me start by telling you a little about the registry.
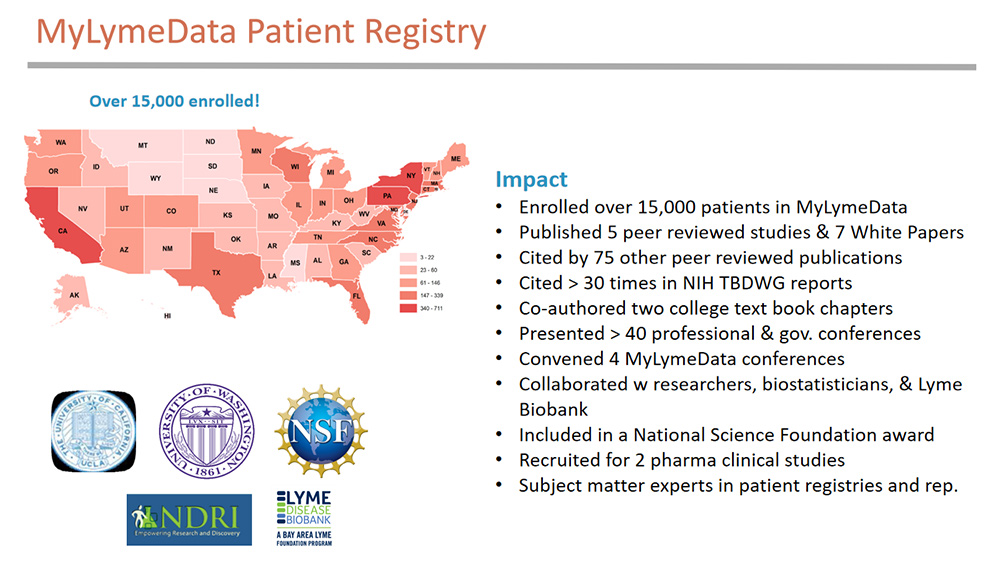
MyLymeData is a patient-led research registry that allows patients to pool their data to accelerate research using real-world evidence. The registry uses the same technology and platform that serves NIH patient registries. It also uses government-validated survey questions to the extent possible. Along the way we’ve formed collaborations with academic researchers, biostatisticians, and the Lyme Disease Biobank, a Bay Area Lyme Foundation Program, and have worked with two industry partners to recruit for clinical trials.
The MyLymeData patient registry was launched just five years ago. During this time, we
- Enrolled over 15,000 patients in MyLymeData
- Were included in a National Science Foundation award
- Published five peer reviewed studies that have been cited by 75 other publications
- Have been cited over 30 times in NIH Tick-Borne Diseases Working Group reports
- Co-authored two college textbook chapters on patient registries and patient representation in healthcare
We are also recognized as subject matter experts in patient registries and patient representation and currently consult with Patient-Centered Outcomes Research Institute (PCORI) through the University of Chicago NORC program.
Lyme disease now has over 476,000 cases annually. But, according to research by Goswami, the number of clinical studies for Lyme disease trails behind leprosy — which has an incidence of less than 200 cases a year. So we need to think of Lyme disease as a research-disadvantaged disease that faces the same challenges and struggles that rare diseases face.
These diseases need innovative team research approaches that allow rapid knowledge generation to accelerate the pace of research. The research cycle illustrated here is derived from Groft’s work with rare diseases.
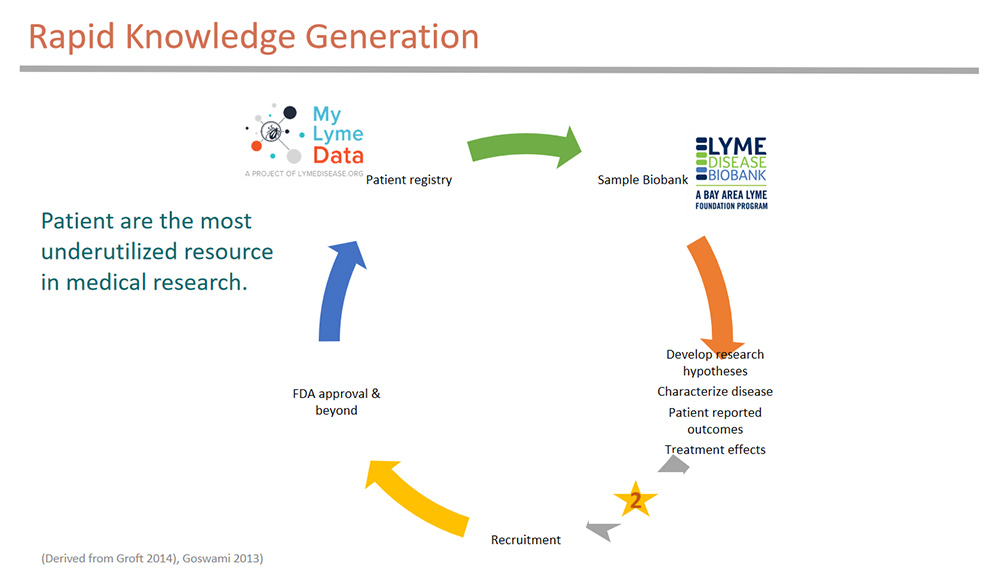
His model suggests forming a patient registry that links with a biorepository — we are collaborating with the Lyme Disease Biobank here. The registry helps develop research hypothesis — here we have published three peer-reviewed publications to better characterize the disease, assess patient-reported outcomes, and analyze treatment effects among patient subgroups……..Join or login below to continue reading.