Bartonellosis: An Emerging Infectious Disease of Zoonotic Importance to Animals and Humans This Emerging Infectious Disease Threatens Both Animals and Humans
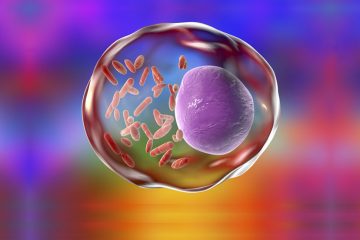
O ver the past three decades, substantial scientific evidence has begun to elucidate the emerging biomedical importance of the genus Bartonella and the disease bartonellosis. Our team of comparative infectious disease researchers at the North Carolina State University Intracellular Pathogens Research Laboratory has generated scientific publications related to bartonellosis in cats, cows, dogs, dolphins, horses, human beings, river otters, sea turtles, sheep, whales, and other wildlife species. The evolving research findings from around the world have left me with the following question:
Is Bartonellosis a modern-day hidden epidemic?
If so, how could a disease of epidemic proportions involving both animals and human patients be missed or remain hidden from diagnosticians, other scientists, physicians, and veterinarians for more than 100 years or perhaps an even a much longer period of medical history?
How much pain and suffering could have been avoided if the “hidden epidemic” called bartonellosis had been discovered sooner?
What has been the emotional, medical, occupational, and financial impact of historically undiscovered Bartonella species (now 38 named or candidatus species have been characterized) infections among patients with bartonellosis throughout the world?
How can an epidemic be hidden?
Hiding an epidemic caused by a genus of bacteria may be easier than one might think. First you would start with a bacterial genus that was essentially not known to exist prior to the 1990s (with the exception of two historically important Bartonella species, Bartonella bacilliformis and Bartonella quintana, the cause of Carrion’s Disease and Trench Fever, respectively). Next, the bacteria in question would have evolved effective mechanisms to avoid immune recognition and thereby behave as a stealth pathogen (i.e., bacteria that can fly under the radar like a stealth bomber).
In addition, Mother Nature might “design” this stealth bacterium with inherent attributes that facilitate effective maintenance of a persistent but relapsing intravascular infection (an infection involving erythrocytes, the immune cells that circulate within the blood, and the cells called endothelium that line the blood vessels), as well as long-standing dermal infections that facilitate transmission to new blood-seeking vectors.
Over time (perhaps tens of thousands of years), these stealth bacteria would differentiate genetically to selectively evolve and preferentially infect a large number of very specific animal reservoir hosts in nature.
Importantly, during evolution, these stealth bacteria would use a broad variety of insects and arthropods to facilitate transmission among the various animal populations.
Opportunistically, those arthropods (flea, sand fly, and tick bites, as examples) would periodically transmit the bacteria directly to humans and other non-reservoir-adapted animals. Finally, to progress from periodic and infrequent infections to an epidemic, you would modify human behaviors to facilitate closer contact among the vector, the reservoir hosts, and human beings. As this epidemic has likely been ongoing throughout the history of mankind, minimal changes in human behaviors were needed to facilitate epidemic numbers of unrecognized infections with various Bartonella species.