LYME SCI: Continuing questions about COVID-19 testing
Testing for COVID-19, the disease caused by the novel coronavirus, has been problematic to say the least. We keep hearing about new and different tests, but as of this writing, they’re not generally available. So, where do we stand now?
Current COVID-19 tests are very specific. This means that if you test positive, you definitely have the infection. (There are almost no false positives.)
However, sensitivity is another matter. Current data on the sensitivity of the CDC’s test is limited, but it appears to range from 66%-80%. Meaning there is a 20-33% chance for a false negative, unfortunately.
In a recent video of Grand Rounds at Stanford University, Dr. Yvonne Maldanado said the current test used by state and federal labs has a 95% confidence interval, if the test is positive. If the test is negative, however, it’s much less conclusive.
Why so many false negatives?
Writing in the New York Times, public health expert Harlan Krumholz, MD, says there are many reasons a test would be falsely negative.
“Perhaps the sampling is inadequate. A common technique requires the collection of nasal secretions far back in the nose — and then rotating the swab several times. That is not an easy procedure to perform or for patients to tolerate. Other possible causes of false negative results are related to laboratory techniques and the substances used in the tests.”
Dr. Krumholz goes on to state: “If you have had likely exposures and symptoms suggest COVID-19 infection, you probably have it — even if your test is negative.”
Testing for COVID-19 is so new there is very little data. However, just like with Lyme disease, some pathogens are not easily found in blood. SARS-CoV-2 (the novel coronavirus) primarily attaches to respiratory pathways, so it is easiest to find in the airways. However, it can also be found in other places.
Where the coronavirus is most easily detected by PCR in patients with COVID-19:
- Bronchoalveolar (lung) fluid 93% positive
- Sputum 72% positive
- Nasal swabs 63% positive
- Pharangeal (throat) swabs 32% positive
- Feces 29% positive
- Blood 1% positive
In the following video, Dr. David Persing, of Cepheid (a company that develops diagnostics) explains some of the challenges involved with rapid testing for coronavirus.
A plan for widespread testing
A new report co-authored by Dr. Gottlieb lays out a road map to reopening the U.S. The report suggests ways the U.S. can dramatically increase its diagnostic testing capacity. The authors state, “same-day, point-of-care diagnostic testing (widely available in outpatient settings) is crucial for identifying cases, including those with asymptomatic and mild infections.”
Asymptomatic and mild cases are particularly dangerous, because these people may unknowingly spread infection to others. One study shows pre-symptomatic and asymptomatic cases may account for up to 30% of the spread of illness.
Having access to rapid, accurate results is critical to controlling this pandemic. Healthcare providers will know when to use full protective equipment. Patients will get treated sooner. Uninfected people will be kept from unnecessary quarantine. Widespread testing will also allow us to identify those who have recovered and presumably no longer at risk of catching it again
What testing is available?
Now that the FDA has removed the red tape allowing universities and commercial manufacturers to innovate, the testing for COVID-19 is literally changing by the day. Check here for a full list of COVID-19 tests with FDA Emergency Use Authorizations
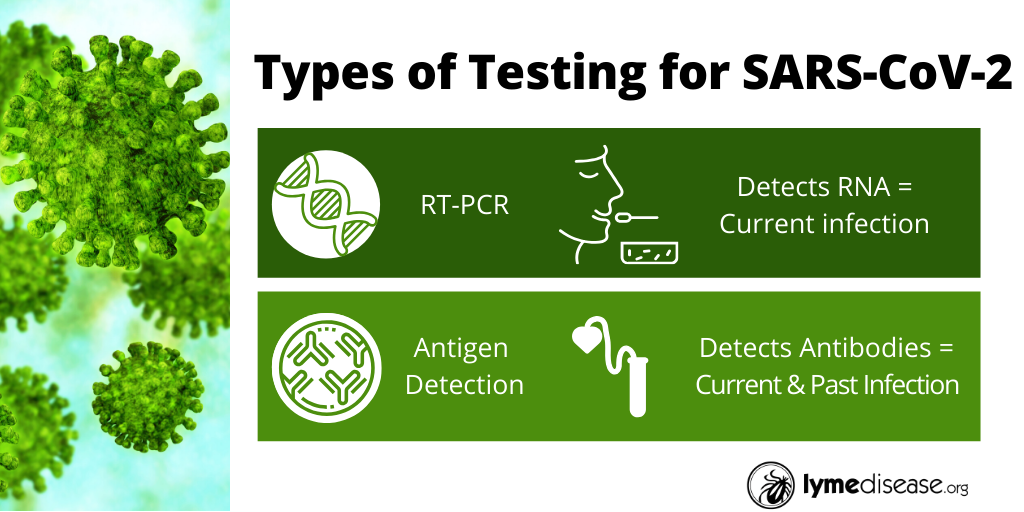
Currently there are two categories of tests. Those that detect the virus itself, and those that detect the immune system’s response to the virus.
The CDC, state and local health departments currently use a test that directly identifies the virus, using Real-Time Reverse Transcriptase (RT) PCR.
Rather than blood, this process tests respiratory secretions collected with a swab. While RT-PCR can detect the virus in four to six hours, many results are delayed due to shipping and processing lags.
Admiral Brett Giroir of the White House COVID-19 Task Force announced the FDA has issued emergency use authorization for a new rapid test that is capable of giving a positive result in five minutes and a negative result in under 15 minutes. Giroir says those tests will be widely available within the next several weeks.
Other tests under development will detect the immune system’s response to the virus. They will be blood-based and will record IgM (early) and IgG (late) antibodies. Because most people don’t develop antibodies until 7-11 days post-infection, these tests will be useful in showing past exposure.
Imagine the benefit if all healthcare providers could know they’ve already gained immunity to the infection. Imagine the benefit if you knew if you or a family member already had it?
Beware: The FDA has not authorized any “at home” testing kits for COVID-19.
Other illnesses that cause symptoms similar to COVID-19
COVID-19 is on everyone’s mind right now. But remember, other illnesses can cause similar symptoms. Obviously, if your symptoms are flu-like, you’ll want to rule out influenza. Respiratory syncytial virus (RSV) also causes difficulty breathing. There are several bacterial infections like strep that commonly affect the airways. Luckily, there are rapid tests for flu, RSV & strep.
And don’t forget babesiosis, a tick-borne illness where patients may develop fatigue, headache, drenching sweats, muscle aches, chest pain, hip pain and shortness of breath often described as “air hunger.”
LymeSci is written by Lonnie Marcum, a Licensed Physical Therapist and mother of a daughter with Lyme. Follow her on Twitter: @LonnieRhea Email her at: lmarcum@lymedisease.org .
Important resources
Virtual Pediatric Grand Rounds (CME): Novel Coronavirus (COVID-19) Update, Yvonne (Bonnie) Maldonado, MD – Stanford School of Medicine
What Should US Clinicians Know About COVID-19? Christina Tan, MD, MPH, state epidemiologist, assistant commissioner, New Jersey Department of Health, Contagion Live
American Enterprise Institute | National coronavirus response: A road map to reopening
CDC | Tests for COVID-19
FDA | Latest COVID-19 Information
FDA | List of Companies Selling Fraudulent Products that Claim to Treat or Prevent COVID-19
References
Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA March 2020
Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: Value of Diagnostic Testing for SARS–CoV-2/COVID-19. ASM Bio, March 2020
Comparing RT-PCR and Chest CT for Diagnosing COVID-19. MD Mag, March 2020
New coronavirus test with results in 45 minutes exceeds expectations, Rutgers says
FDA Authorizes New Two-Minute Serological Test Kit to Detect Novel Coronavirus
Presymptomatic Transmission of SARS-CoV-2 — Singapore, January 23 – March 16, 2020. CDC, MMWR, Early Release, April 2020




















We invite you to comment on our Facebook page.
Visit LymeDisease.org Facebook Page